Antibody data
- Antibody Data
- Antigen structure
- References [9]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-1038-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD103 (Integrin alpha E) Monoclonal Antibody (B-Ly7), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The B-Ly7 monoclonal antibody reacts with human CD103, the alpha E integrin. CD103 non-covalently associates with integrin beta 7. CD103 is expressed mainly on intraepithelial lymphocytes and a small subset of peripheral lymphocytes. CD103 is also expressed by hairy cell leukemia (HCL) and by some chronic B cell lymphocytic leukemias. In vitro stimulation of human T cells with mitogens induces upregulation of CD103. Epithelial cell antigen, E-cadherin, binds to CD103 and mediates homing of lymphocytes to the intestinal epithelium. Applications Reported: B-Ly7 has been reported for use in flow cytometric analysis, and immunohistochemical staining of frozen tissue sections. Applications Tested: The B-Ly7 antibody has been tested by flow cytometric analysis of stimulated normal human peripheral blood cells. This can be used at less than or equal to 0.5 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- B-Ly7
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells.
Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors.
Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin αvβ8.
Optimization of mass cytometry sample cryopreservation after staining.
Tissue Distribution of Memory T and B Cells in Rhesus Monkeys following Influenza A Infection.
RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection.
Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques.
DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues.
Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells.
Duhen R, Ballesteros-Merino C, Frye AK, Tran E, Rajamanickam V, Chang SC, Koguchi Y, Bifulco CB, Bernard B, Leidner RS, Curti BD, Fox BA, Urba WJ, Bell RB, Weinberg AD
Nature communications 2021 Feb 16;12(1):1047
Nature communications 2021 Feb 16;12(1):1047
Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors.
Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, Chang SC, Grunkemeier G, Leidner R, Bell RB, Weinberg AD
Nature communications 2018 Jul 13;9(1):2724
Nature communications 2018 Jul 13;9(1):2724
Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin αvβ8.
Fenton TM, Kelly A, Shuttleworth EE, Smedley C, Atakilit A, Powrie F, Campbell S, Nishimura SL, Sheppard D, Levison S, Worthington JJ, Lehtinen MJ, Travis MA
Mucosal immunology 2017 May;10(3):624-634
Mucosal immunology 2017 May;10(3):624-634
Optimization of mass cytometry sample cryopreservation after staining.
Sumatoh HR, Teng KW, Cheng Y, Newell EW
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2017 Jan;91(1):48-61
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2017 Jan;91(1):48-61
Tissue Distribution of Memory T and B Cells in Rhesus Monkeys following Influenza A Infection.
Pichyangkul S, Yongvanitchit K, Limsalakpetch A, Kum-Arb U, Im-Erbsin R, Boonnak K, Thitithayanont A, Jongkaewwattana A, Wiboon-ut S, Mongkolsirichaikul D, Mahanonda R, Spring M, Chuang I, Mason CJ, Saunders DL
Journal of immunology (Baltimore, Md. : 1950) 2015 Nov 1;195(9):4378-86
Journal of immunology (Baltimore, Md. : 1950) 2015 Nov 1;195(9):4378-86
RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection.
Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EHC, Sykes A, Maybeno M, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C
Nature communications 2015 Dec 21;6:10224
Nature communications 2015 Dec 21;6:10224
Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques.
Canary LA, Vinton CL, Morcock DR, Pierce JB, Estes JD, Brenchley JM, Klatt NR
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 15;190(6):2959-65
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 15;190(6):2959-65
DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues.
Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, Håkansson UK, Moita LF, Agace WW, Bonnet D, Reis e Sousa C
Blood 2012 Jun 21;119(25):6052-62
Blood 2012 Jun 21;119(25):6052-62
Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells.
Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, Reis e Sousa C
The Journal of experimental medicine 2010 Jun 7;207(6):1261-71
The Journal of experimental medicine 2010 Jun 7;207(6):1261-71
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
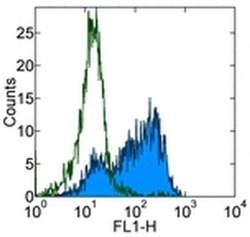
- Experimental details
- Staining of 3-day PHA-stimulated normal human peripheral blood cells with 0.25 µg of Mouse IgG1 K Isotype Control Purified (Product # 14-4714-82) (open histogram) or 0.25 µg of Anti-Human CD103 (Integrin alpha E) Purified (filled histogram) followed by Anti-Mouse IgG FITC (Product # 11-4011-85). Total viable cells were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
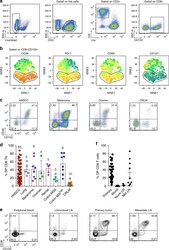
- Experimental details
- Fig. 1 Tumor-infiltrating CD103 + CD8 T cells coexpress the ectonucleotidase CD39. a Representative gating strategy for the flow cytometric analysis of tumor-infiltrating CD8 T cells (CD8 TIL) for a HNSCC patient. Numbers in plots indicate the percent cells in respective gates. b viSNE analysis of CD103 + CD8 TILs as shown in a . The gate identifies the CD103 + CD8 T cells with the highest expression of CD39. The gate is applied to all plots showing expression levels of PD-1, CD69, and CD127. c Flow cytometric analysis of CD8 TIL isolated from representative HNSCC, melanoma, ovarian cancer, and CRLM patients. Numbers in each quadrant indicate percent cells positive for CD39 and/or CD103 on CD3 + CD8 + T cells. d Summary of the frequency of CD39 + CD103 + (DP) CD8 TILs among patients with different solid malignancies. Shown are 65 HNSCC, 5 lung cancers, 8 melanomas, 4 ovarian cancers, 5 rectal cancers, 5 MSS colon cancers, 8 MSI high colon cancers, and 10 CRLM. e Flow cytometric analysis of the percentage of DP CD8 T cells in peripheral blood, uninvolved LN, primary tumor, and metastatic LN from a representative HNSCC patient. Numbers in each quadrant indicate percent cells positive for CD39 and/or CD103 on CD3 + CD8 + T cells. Data are representative of 41 HNSCC patients analyzed (7 patients for uninvolved LN and 9 patients for Met LN). Percentages are summarized in f . Small horizontal lines indicate mean +- SEM
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
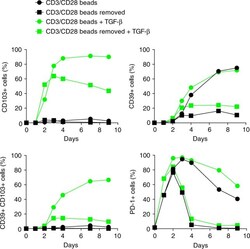
- Experimental details
- Fig. 4 TGF-beta and sustained TCR stimulation are required for CD103 and CD39 upregulation on CD8 T cells. Kinetics of CD39, CD103, and PD-1 expression on sorted naive CD8 T cells from peripheral blood after in vitro activation. Cells were stimulated with CD3/CD28-coated beads at a bead:T-cell ratio of 1:2 in the presence or absence of TGF-beta1 (2 ng/ml), and expression of CD39, CD103, and PD-1 was analyzed by flow cytometry at the indicated time points. Data are from one representative healthy donor
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
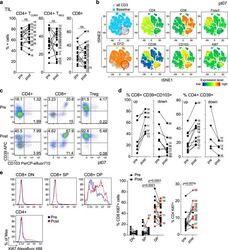
- Experimental details
- Fig. 2 Changes in TIL composition after OX40 administration. TIL from a pretreatment biopsy and a surgical specimen after OX40 therapy were assessed for lymphocyte composition and activation markers. The gating strategy is outlined in Supplementary Fig. 3 . a Percentages of CD4+ Tconv cells, CD4+ Treg cells, and CD8+ T cells in each patient before and after OX40 administration, N = 17 patients. b tSNE analysis of the pre and post specimens from patient HNOX07, gated on CD3+ cells. Blue represents the baseline sample, orange the day of surgery sample and gray is the concatenated file. The red circle indicates the population of cells expressing both CD103 and CD39. tSNE analysis was performed on N = 4 patients, one representative patient is shown here, two more patients are shown in Supplementary Fig. 4 . c Flow cytometric analysis of the expression of CD103 and CD39 in CD4+ Tconv cells, CD8+ cells, and CD4+ Treg cells in one immune-responding head and neck squamous cell carcinoma (HNSCC) patient pre- and post OX40 therapy. d Summary of the flow cytometric analysis in (c), left panel depicts CD8+ CD103+ CD39+ T cells and the right panel depicts CD4+ CD39+ T cells; patients with an increase are shown on the left, patients with a decrease are on the right. e Expression of Ki-67 was assessed among memory CD4+ TIL and CD8+ TIL subsets (DN, SP, and DP) in biopsy (pre) and DOS (post) tissue ( N = 17 patients). Blue histograms indicate pre, red indicate post tissues. The left graph sh
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
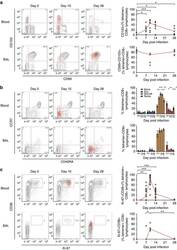
- Experimental details
- Figure 6 RSV-specific CD8+ T cells in BAL display a distinctive resident memory phenotype. Tetramer+ CD8+ T cells in blood and BAL were co-stained for markers to assess their differentiation status. ( a ) CD69 and CD103 as canonical markers of resident memory CD8+ T cells are shown in blood ( n =9) and BAL ( n =5) from infected volunteers. Significant P values for two-tailed Wilcoxon matched pairs tests in blood compared with baseline are shown (day 7, P =0.0313; day 10, P =0.0039; day 14, P =0.0313; and day 28, P =0.0313). ( b ) Memory markers CD45RA and CCR7 are shown in blood ( n =19) and BAL ( n =8). Mean+-significant P values for two-tailed Wilcoxon matched pairs tests compared with baseline are shown in blood for T-effector/effector memory cells (day 7, P =0.0034; day 10, P =0.0002; day 14, P =0.0002; day 28, P =0.0067) and effector memory T cells re-expressing CD45RA (day 7, P =0.0443; day 10, P =0.0025; day 14, P =0.0003; day 28, P =0.0135). ( c ) Proliferation and activation markers Ki-67 and CD38 are shown in blood ( n =19) and BAL ( n =8). Significant P values for two-tailed Wilcoxon matched pairs tests are shown compared with baseline in blood (day 7, P =0.0025; day 10, P =0.0001; and day 14, P =0.0005) and BAL (day 7 versus day 10, P =0.0444; and day 10 versus day 28, P =0.0022 as no Ki-67+CD38+ cells were found in any baseline samples). Throughout, representative plots from a single subject at day 0, 10 and 28 post infection are shown with tetramer+ cells as red
 Explore
Explore Validate
Validate Learn
Learn Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry