MA3-029
antibody from Invitrogen Antibodies
Targeting: CDC37
P50CDC37
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [17]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunohistochemistry [2]
- Flow cytometry [6]
- Other assay [10]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-029 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Cdc37 Monoclonal Antibody (C1)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA3-029 detects Cdc37 from hamster, human, mouse and rat cells and tissues. MA3-029 has been successfully used in Western blot, FACS, immunofluorescence, immunocytochemistry and immunoprecipitation (under denaturing conditions) procedures. By Western blot, this antibody recognizes a single 50 kDa protein representing Cdc37 in HeLa cell extract. Immunofluorescence staining of Cdc37 in Hepa1 cells with MA3-029 results in mostly cytoplasmic staining concentrated around the nuclear membrane. This antibody detects some weak non-specific bands in Jurkat, A431 and NIH-3T3 cell lysates. The MA3-029 antigen is purified mouse Cdc37 fusion protein.
- Reactivity
- Human, Mouse, Rat, Hamster
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- C1
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references The Alpha Isoform of Heat Shock Protein 90 and the Co-chaperones p23 and Cdc37 Promote Opioid Anti-nociception in the Brain.
PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm.
Heat shock protein 90 controls HIV-1 reactivation from latency.
Hsp90 cochaperones p23 and FKBP4 physically interact with hAgo2 and activate RNA interference-mediated silencing in mammalian cells.
A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates.
Functioning of the Hsp90 machine in chaperoning checkpoint kinase I (Chk1) and the progesterone receptor (PR).
Functioning of the Hsp90 machine in chaperoning checkpoint kinase I (Chk1) and the progesterone receptor (PR).
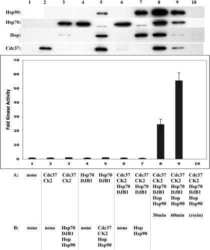
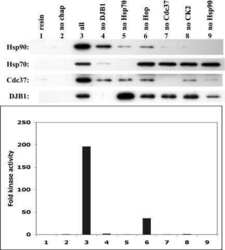
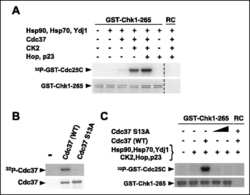
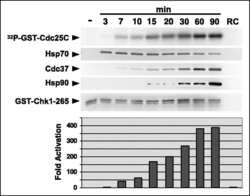
Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones.
Evidence for chaperone heterocomplexes containing both Hsp90 and VCP.
Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer.
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37.
Cdc37 is a molecular chaperone with specific functions in signal transduction.
Cdc37 is a molecular chaperone with specific functions in signal transduction.
Interaction between Cdc37 and Cdk4 in human cells.
Immunolocalization of cellular retinoic acid binding protein to Müller cells and/or a subpopulation of GABA-positive amacrine cells in retinas of different species.
Lei W, Duron DI, Stine C, Mishra S, Blagg BSJ, Streicher JM
Frontiers in molecular neuroscience 2019;12:294
Frontiers in molecular neuroscience 2019;12:294
PDLIM1 inhibits NF-κB-mediated inflammatory signaling by sequestering the p65 subunit of NF-κB in the cytoplasm.
Ono R, Kaisho T, Tanaka T
Scientific reports 2015 Dec 18;5:18327
Scientific reports 2015 Dec 18;5:18327
Heat shock protein 90 controls HIV-1 reactivation from latency.
Anderson I, Low JS, Weston S, Weinberger M, Zhyvoloup A, Labokha AA, Corazza G, Kitson RA, Moody CJ, Marcello A, Fassati A
Proceedings of the National Academy of Sciences of the United States of America 2014 Apr 15;111(15):E1528-37
Proceedings of the National Academy of Sciences of the United States of America 2014 Apr 15;111(15):E1528-37
Hsp90 cochaperones p23 and FKBP4 physically interact with hAgo2 and activate RNA interference-mediated silencing in mammalian cells.
Pare JM, LaPointe P, Hobman TC
Molecular biology of the cell 2013 Aug;24(15):2303-10
Molecular biology of the cell 2013 Aug;24(15):2303-10
A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates.
Taherian A, Krone PH, Ovsenek N
Biochemistry and cell biology = Biochimie et biologie cellulaire 2008 Feb;86(1):37-45
Biochemistry and cell biology = Biochimie et biologie cellulaire 2008 Feb;86(1):37-45
Functioning of the Hsp90 machine in chaperoning checkpoint kinase I (Chk1) and the progesterone receptor (PR).
Felts SJ, Karnitz LM, Toft DO
Cell stress & chaperones 2007 Winter;12(4):353-63
Cell stress & chaperones 2007 Winter;12(4):353-63
Functioning of the Hsp90 machine in chaperoning checkpoint kinase I (Chk1) and the progesterone receptor (PR).
Felts SJ, Karnitz LM, Toft DO
Cell stress & chaperones 2007 Winter;12(4):353-63
Cell stress & chaperones 2007 Winter;12(4):353-63
Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones.
Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM
The Journal of biological chemistry 2006 Feb 3;281(5):2989-98
The Journal of biological chemistry 2006 Feb 3;281(5):2989-98
Evidence for chaperone heterocomplexes containing both Hsp90 and VCP.
Prince T, Shao J, Matts RL, Hartson SD
Biochemical and biophysical research communications 2005 Jun 17;331(4):1331-7
Biochemical and biophysical research communications 2005 Jun 17;331(4):1331-7
Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer.
Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C
Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005 Feb 20;23(6):1078-87
Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005 Feb 20;23(6):1078-87
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL
Biochemistry 2000 Jun 27;39(25):7631-44
Biochemistry 2000 Jun 27;39(25):7631-44
p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules.
Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL
Biochemistry 2000 Jun 27;39(25):7631-44
Biochemistry 2000 Jun 27;39(25):7631-44
A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37.
Perdew GH, Wiegand H, Vanden Heuvel JP, Mitchell C, Singh SS
Biochemistry 1997 Mar 25;36(12):3600-7
Biochemistry 1997 Mar 25;36(12):3600-7
Cdc37 is a molecular chaperone with specific functions in signal transduction.
Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S
Genes & development 1997 Jul 15;11(14):1775-85
Genes & development 1997 Jul 15;11(14):1775-85
Cdc37 is a molecular chaperone with specific functions in signal transduction.
Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S
Genes & development 1997 Jul 15;11(14):1775-85
Genes & development 1997 Jul 15;11(14):1775-85
Interaction between Cdc37 and Cdk4 in human cells.
Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta GF, Gyuris J
Oncogene 1997 Apr 24;14(16):1999-2004
Oncogene 1997 Apr 24;14(16):1999-2004
Immunolocalization of cellular retinoic acid binding protein to Müller cells and/or a subpopulation of GABA-positive amacrine cells in retinas of different species.
Milam AH, De Leeuw AM, Gaur VP, Saari JC
The Journal of comparative neurology 1990 Jun 1;296(1):123-9
The Journal of comparative neurology 1990 Jun 1;296(1):123-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
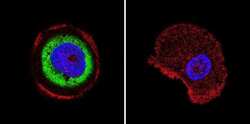
- Experimental details
- Immunofluorescent analysis of Cdc37 in A431 cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:200 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Cdc37 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
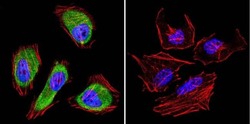
- Experimental details
- Immunofluorescent analysis of Cdc37 in HeLa cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:200 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Cdc37 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
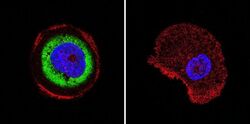
- Experimental details
- Immunofluorescent analysis of Cdc37 in A431 cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:200 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Cdc37 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
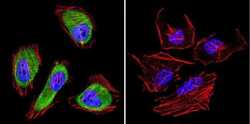
- Experimental details
- Immunofluorescent analysis of Cdc37 in HeLa cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:200 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Cdc37 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
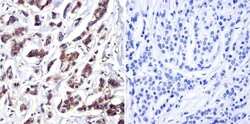
- Experimental details
- Immunohistochemistry was performed on cancer biopsies of deparaffinized human breast carcinoma. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a Mouse Monoclonal Antibody recognizing Cdc37 (Product # MA3-029) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
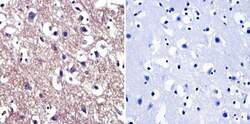
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized human brain tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a Mouse Monoclonal Antibody recognizing Cdc37 (Product # MA3-029) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
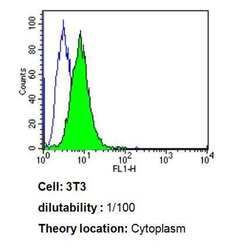
- Experimental details
- Flow cytometry analysis of Cdc37 in 3T3 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:100 for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
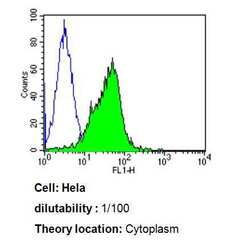
- Experimental details
- Flow cytometry analysis of Cdc37 in Hela cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:100 for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
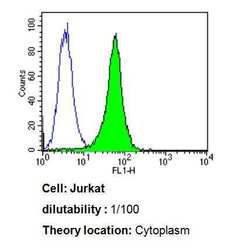
- Experimental details
- Flow cytometry analysis of Cdc37 in Jurkat cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:100 for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
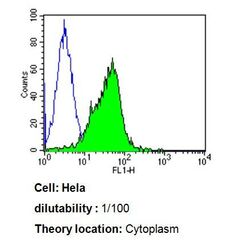
- Experimental details
- Flow cytometry analysis of Cdc37 in Hela cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:100 for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
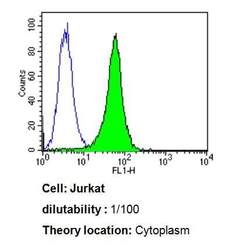
- Experimental details
- Flow cytometry analysis of Cdc37 in Jurkat cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:100 for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
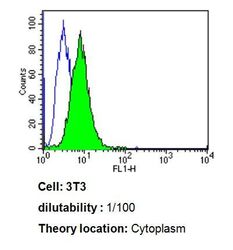
- Experimental details
- Flow cytometry analysis of Cdc37 in 3T3 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with Cdc37 monoclonal antibody (Product # MA3-029) at a dilution of 1:100 for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
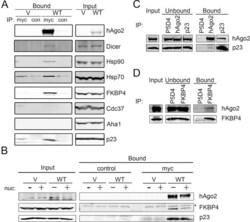
- Experimental details
- FIGURE 1: Human Argonaute2 exists in complex with Dicer, Hsp90, and the cochaperones FKBP4 and p23. (A) HeLa cells transiently transfected with pcDNA3/myc-hAgo2 (WT) or empty pcDNA3 (V) were lysed 16 h posttransfection and subjected to immunoprecipitation (IP) using Sepharose beads coated with monoclonal anti-myc antibodies (myc) or anti-VSVG (con) as a control. Total cell lysate (Input) and bound fractions were subjected to SDS-PAGE and immunoblotted for hAgo2, Dicer, Hsp90, Hsp70, and cochaperones as indicated. (B) Immunoprecipitations performed as in A were treated with or without the nuclease Benzonase (nuc) for 15 min before addition of Sepharose beads. (C, D) Lysates of untransfected HeLa cells were subjected to immunoprecipitation using monoclonal antibodies against hAgo2, p23, FKBP4, or VSV-G (P5D4) as a control. Total cell lysate (Input) and unbound and bound fractions were subjected to SDS-PAGE and immunoblotted for hAgo2, p23, and FKBP4 as indicated. Nonspecific band marked with an asterisk.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
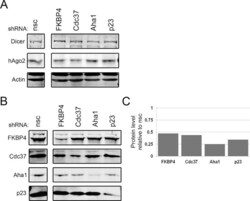
- Experimental details
- FIGURE 3: Knockdown of Hsp90 cochaperones does not affect the level of RNAi effector proteins. (A) HeLa cells were harvested 72 h after transient cotransfection with pLKO.1 vectors expressing shRNAs against hAgo2, FKBP4, Cdc37, Aha1, p23, or a nonsilencing control (nsc) as a control. Lysates were subjected to SDS-PAGE, and the levels of the RNAi effector proteins Dicer and hAgo2 were determined by immunoblot analysis. (B) Lysates from A were immunoblotted for the targeted cochaperones FKBP4, Cdc37, Aha1, and p23. (C) Signal intensities for FKBP4, Cdc37, Aha1, and p23 were quantitated for each knockdown. The bar graph indicates the average level of expression of the shRNA-targeted protein remaining 72 h posttransfection relative to the nonsilencing control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
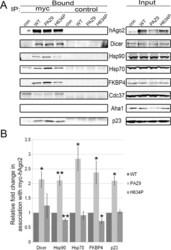
- Experimental details
- FIGURE 4: Inability to load small RNAs increases association between hAgo2, Hsp90, and cochaperones. (A) HeLa cells transiently transfected with pcDNA3/myc-hAgo2 (WT), pcDNA3/myc-hAgo2-PAZ9 (PAZ9), pcDNA3/myc-hAgo2-H634P (H634P), or empty pcDNA3 (con) were lysed 16 h posttransfection and subjected to immunoprecipitation using Sepharose beads coated with monoclonal anti-myc antibodies (myc) or anti-VSVG (control) as a control. Total cell lysate (Input) and bound fractions were subjected to SDS-PAGE and immunoblotted for hAgo2, Dicer, Hsp90, Hsp70, and cochaperones as indicated. (B) Signal intensities for Dicer, Hsp90, Hsp70, FKBP4, and p23 were quantitated and normalized to level of immunoprecipitated hAgo2. The bar graph indicates the fold change in the amount of each coimmunoprecipitated hAgo2-binding partner analyzed relative to the amount associated with wild-type hAgo2. Error bars indicate SE. * p < 0.05; ** p < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
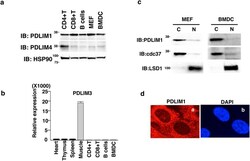
- Experimental details
- Figure 1 PDLIM1 is a cytoplasmic protein expressed in dendritic cells. ( a ) Western blot analysis of PDLIM1 and PDLIM4 expression in primary immune cells. Whole cell lysates were subjected to immunoblot (IB) with anti-PDLIM1, PDLIM4 and HSP90 antibodies. Western blots are representative of at least three independent experiments. ( b ) Real-time RT-PCR analysis of PDLIM3 expression in mouse tissues and primary immune cells. Data are means +- SD from at least three independent experiments. ( c ) Bone-marrow derived dendritic cells (BMDC) and mouse embryonic fibroblasts (MEF) were fractionated into cytoplasmic (C) and nuclear (N) fractions, which were then analyzed by Western blotting with anti-PDLIM1 antibody. The purity of the fractionations was confirmed by anti-cdc37 (cytoplasm) or anti-LSD1 (nucleus) antibody. Western blots are representative of at least three independent experiments. ( d ) Cytoplasmic localization of PDLIM1 in MEF was determined by indirect immunofluorescence with an in situ proximity-ligation assay. DAPI, nuclear staining. Original magnification, x630. Data are representative of at least three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
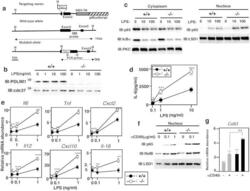
- Figure 6 Enhanced p65-mediated inflammatory responses in Pdlim1 -/- dendritic cells. ( a ) Schematic diagram of the Pdlim1 targeting vector, wild-type allele and mutated allele for generation of PDLIM1-deficient mice. The arrowheads represent the primers used for screening by PCR analysis and the probe used for Southern blot analysis is shown as a closed bar. Abbreviation for restriction sites is as follows: H, HindIII . The two headed arrows indicate the size of the HindIII fragment in the WT and mutant allele. ( b ) Western blot analysis of PDLIM1 expression in cytoplasmic extracts of bone marrow-derived dendritic cells from Pdlim1 +/+ and Pdlim1 -/- mice, either untreated or treated for 1 h with LPS. Western blots are representative of at least three independent experiments. ( c ) Immunoblot of cytoplasmic and nuclear extracts of Pdlim1 +/+ and Pdlim1 -/- dendritic cells, either untreated or treated for 1 h with LPS (10 and 100 ng/ml), analyzed with the indicated antibodies (IB, left margin). Western blots are representative of at least three independent experiments. ( d ) Enzyme-linked immunosorbent assay (ELISA) of IL-6 in culture supernatants of Pdlim1 +/+ and Pdlim1 -/- dendritic cells, stimulated for 24 h with the indicated concentrations of LPS. Data are means +- SD from at least three independent experiments. *P < 0.05; ***P < 0.001. ( e ) Real-time RT-PCR analysis of LPS-inducible genes in Pdlim1 +/+ and Pdlim1 -/- dendritic cells, unstimulated or stimulated with L
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
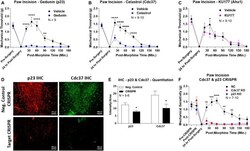
- Figure 4 Identification of the co-chaperones p23 and Cdc37 as promoters of opioid anti-nociception in the brain. Male CD-1 mice used for all experiments, data reported as the mean +- SEM, with sample sizes of mice/group noted in each graph. (A-C) Ten nanomoles of Gedunin (p23, A ), 10 nmol of Celastrol (Cdc37, B ), or 0.1 nmol of KU177 (Aha1, C ) or Vehicle control injected icv concurrently with paw incision surgery with a 24 h recovery, followed by 3.2 mg/kg morphine sc . Experiments performed with two technical replicates for each drug. *,**,**** p < 0.05, 0.01, 0.0001 vs. same time point inhibitor treatment group by two-way ANOVA with Fisher's Least Significant Difference post hoc test. (D) p23, Cdc37, or Negative Control CRISPR-treated mice with icv delivery of constructs analyzed by IHC for target knockdown on day 10. Representative images shown from the PRN. Both targets (p23 - red, Cdc37 - green) have a similar staining pattern to Hsp90alpha, and both are clearly reduced by CRISPR treatment. (E) Data from (D) for all mice quantitated as described in the ""Materials and Methods"" section. All mice treated in one technical replicate, with staining and analysis of the resulting tissue performed in more than one technical replicate. * p < 0.05 vs. same target Negative Control group by Unpaired 2-Tailed t -test. CRISPR treatment reduced p23 signal by 36.3% and Cdc37 by 46.0%. (F) CRISPR-treated mice had paw incision surgery performed on day 9, with injection of 3.2 mg/kg mo
 Explore
Explore Validate
Validate Learn
Learn