MA1-91124
antibody from Invitrogen Antibodies
Targeting: ADGRE1
EMR1, TM7LN3
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Immunoelectron microscopy
Immunoelectron microscopy Radioimmunoassay
Radioimmunoassay Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [41]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Other assay [12]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-91124 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- F4/80 Monoclonal Antibody (CI:A3-1)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Positive control of mouse macrophages suggested. MA1-91124 detects a band of approximately 160 kDa due to the protein being heavily glycosylated, predicted molecular weight of precursor protein is 102 kDa. Store product as a concentrated solution. Centrifuge briefly prior to opening the vial. This antibody recognizes murine F4/80 antigen which shares 68% overall amino acid identity with human EGF module-containing mucin-like hormone receptor 1 (EMR1).
- Reactivity
- Human, Mouse, Rat
- Host
- Rat
- Isotype
- IgG
- Antibody clone number
- CI:A3-1
- Vial size
- 250 μg
- Concentration
- 1 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Myometrial-derived CXCL12 promotes lipopolysaccharide induced preterm labour by regulating macrophage migration, polarization and function in mice.
RhoA within myofibers controls satellite cell microenvironment to allow hypertrophic growth.
Chronic circadian shift leads to adipose tissue inflammation and fibrosis.
Extracellular vesicle-encapsulated microRNA-23a from dorsal root ganglia neurons binds to A20 and promotes inflammatory macrophage polarization following peripheral nerve injury.
Epicardial placement of human MSC-loaded fibrin sealant films for heart failure: Preclinical efficacy and mechanistic data.
A New Gorilla Adenoviral Vector with Natural Lung Tropism Avoids Liver Toxicity and Is Amenable to Capsid Engineering and Vector Retargeting.
Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement.
Macrophage-Specific Hypoxia-Inducible Factor-1α Contributes to Impaired Autophagic Flux in Nonalcoholic Steatohepatitis.
Gene activation of CEBPA using saRNA: preclinical studies of the first in human saRNA drug candidate for liver cancer.
Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation.
The common parasite Toxoplasma gondii induces prostatic inflammation and microglandular hyperplasia in a mouse model.
Tubular overexpression of gremlin induces renal damage susceptibility in mice.
Inflammatory neovascularization during graft-versus-host disease is regulated by αv integrin and miR-100.
Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes.
Antiviral CD8+ T cell effector activities in situ are regulated by target cell type.
The manganese superoxide dismutase mimetic, M40403, protects adult mice from lethal total body irradiation.
Absence of IL-23p19 in donor allogeneic cells reduces mortality from acute GVHD.
Conventional bone marrow-derived dendritic cells contribute to toll-like receptor-independent production of alpha/beta interferon in response to inactivated parapoxvirus ovis.
IL-4Ralpha responsiveness of non-CD4 T cells contributes to resistance in schistosoma mansoni infection in pan-T cell-specific IL-4Ralpha-deficient mice.
Murine neutrophils present Class II restricted antigen.
Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine.
Postburn monocytes are the major producers of TNF-alpha in the heterogeneous splenic macrophage population.
Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine.
Beta cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice.
CD40/CD154 interactions are required for the optimal maturation of skin-derived APCs and the induction of helminth-specific IFN-gamma but not IL-4.
Downregulation of mitogen-activated protein kinases by the Bordetella bronchiseptica Type III secretion system leads to attenuated nonclassical macrophage activation.
Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly.
Viral induction of central nervous system innate immune responses.
Helminth infection protects mice from anaphylaxis via IL-10-producing B cells.
Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice.
Protection against progressive leishmaniasis by IFN-beta.
Lung cell responses to M. tuberculosis in genetically susceptible and resistant mice following intratracheal challenge.
Control of Leishmania major in the absence of Tyk2 kinase.
Quantitative analysis by flow cytometry of interstitial cells of Cajal, pacemakers, and mediators of neurotransmission in the gastrointestinal tract.
Mycobacterial dissemination and cellular responses after 1-lobe restricted tuberculosis infection of genetically susceptible and resistant mice.
The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response.
Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase.
A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection.
In vitro and in vivo regulation by macrophage migration inhibitory factor (MIF) of expression of MHC-II, costimulatory, adhesion, receptor, and cytokine molecules.
Tumor-infiltrating macrophages induce apoptosis in activated CD8(+) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide.
CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ.
Zhang L, Mamillapalli R, Habata S, McAdow M, Taylor HS
Journal of cellular and molecular medicine 2022 May;26(9):2566-2578
Journal of cellular and molecular medicine 2022 May;26(9):2566-2578
RhoA within myofibers controls satellite cell microenvironment to allow hypertrophic growth.
Noviello C, Kobon K, Delivry L, Guilbert T, Britto F, Julienne F, Maire P, Randrianarison-Huetz V, Sotiropoulos A
iScience 2022 Jan 21;25(1):103616
iScience 2022 Jan 21;25(1):103616
Chronic circadian shift leads to adipose tissue inflammation and fibrosis.
Xiong X, Lin Y, Lee J, Paul A, Yechoor V, Figueiro M, Ma K
Molecular and cellular endocrinology 2021 Feb 5;521:111110
Molecular and cellular endocrinology 2021 Feb 5;521:111110
Extracellular vesicle-encapsulated microRNA-23a from dorsal root ganglia neurons binds to A20 and promotes inflammatory macrophage polarization following peripheral nerve injury.
Zhang Y, Liu J, Wang X, Zhang J, Xie C
Aging 2021 Feb 17;13(5):6752-6764
Aging 2021 Feb 17;13(5):6752-6764
Epicardial placement of human MSC-loaded fibrin sealant films for heart failure: Preclinical efficacy and mechanistic data.
Fields L, Ito T, Kobayashi K, Ichihara Y, Podaru MN, Hussain M, Yamashita K, Machado V, Lewis-McDougall F, Suzuki K
Molecular therapy : the journal of the American Society of Gene Therapy 2021 Aug 4;29(8):2554-2570
Molecular therapy : the journal of the American Society of Gene Therapy 2021 Aug 4;29(8):2554-2570
A New Gorilla Adenoviral Vector with Natural Lung Tropism Avoids Liver Toxicity and Is Amenable to Capsid Engineering and Vector Retargeting.
Lu ZH, Dmitriev IP, Brough DE, Kashentseva EA, Li J, Curiel DT
Journal of virology 2020 May 4;94(10)
Journal of virology 2020 May 4;94(10)
Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement.
Kalinski AL, Yoon C, Huffman LD, Duncker PC, Kohen R, Passino R, Hafner H, Johnson C, Kawaguchi R, Carbajal KS, Jara JS, Hollis E, Geschwind DH, Segal BM, Giger RJ
eLife 2020 Dec 2;9
eLife 2020 Dec 2;9
Macrophage-Specific Hypoxia-Inducible Factor-1α Contributes to Impaired Autophagic Flux in Nonalcoholic Steatohepatitis.
Wang X, de Carvalho Ribeiro M, Iracheta-Vellve A, Lowe P, Ambade A, Satishchandran A, Bukong T, Catalano D, Kodys K, Szabo G
Hepatology (Baltimore, Md.) 2019 Feb;69(2):545-563
Hepatology (Baltimore, Md.) 2019 Feb;69(2):545-563
Gene activation of CEBPA using saRNA: preclinical studies of the first in human saRNA drug candidate for liver cancer.
Reebye V, Huang KW, Lin V, Jarvis S, Cutilas P, Dorman S, Ciriello S, Andrikakou P, Voutila J, Saetrom P, Mintz PJ, Reccia I, Rossi JJ, Huber H, Habib R, Kostomitsopoulos N, Blakey DC, Habib NA
Oncogene 2018 Jun;37(24):3216-3228
Oncogene 2018 Jun;37(24):3216-3228
Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation.
Vasamsetti SB, Florentin J, Coppin E, Stiekema LCA, Zheng KH, Nisar MU, Sembrat J, Levinthal DJ, Rojas M, Stroes ESG, Kim K, Dutta P
Immunity 2018 Jul 17;49(1):93-106.e7
Immunity 2018 Jul 17;49(1):93-106.e7
The common parasite Toxoplasma gondii induces prostatic inflammation and microglandular hyperplasia in a mouse model.
Colinot DL, Garbuz T, Bosland MC, Wang L, Rice SE, Sullivan WJ Jr, Arrizabalaga G, Jerde TJ
The Prostate 2017 Jul;77(10):1066-1075
The Prostate 2017 Jul;77(10):1066-1075
Tubular overexpression of gremlin induces renal damage susceptibility in mice.
Droguett A, Krall P, Burgos ME, Valderrama G, Carpio D, Ardiles L, Rodriguez-Diez R, Kerr B, Walz K, Ruiz-Ortega M, Egido J, Mezzano S
PloS one 2014;9(7):e101879
PloS one 2014;9(7):e101879
Inflammatory neovascularization during graft-versus-host disease is regulated by αv integrin and miR-100.
Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont RA, Braun F, Fani M, Riesner K, Prinz G, Hechinger AK, Gerlach UV, Dierbach H, Penack O, Schmitt-Gräff A, Finke J, Weber WA, Zeiser R
Blood 2013 Apr 25;121(17):3307-18
Blood 2013 Apr 25;121(17):3307-18
Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes.
Sekine C, Koyanagi A, Koyama N, Hozumi K, Chiba S, Yagita H
Arthritis research & therapy 2012 Mar 5;14(2):R45
Arthritis research & therapy 2012 Mar 5;14(2):R45
Antiviral CD8+ T cell effector activities in situ are regulated by target cell type.
Hufford MM, Kim TS, Sun J, Braciale TJ
The Journal of experimental medicine 2011 Jan 17;208(1):167-80
The Journal of experimental medicine 2011 Jan 17;208(1):167-80
The manganese superoxide dismutase mimetic, M40403, protects adult mice from lethal total body irradiation.
Thompson JS, Chu Y, Glass J, Tapp AA, Brown SA
Free radical research 2010 May;44(5):529-40
Free radical research 2010 May;44(5):529-40
Absence of IL-23p19 in donor allogeneic cells reduces mortality from acute GVHD.
Thompson JS, Chu Y, Glass JF, Brown SA
Bone marrow transplantation 2010 Apr;45(4):712-22
Bone marrow transplantation 2010 Apr;45(4):712-22
Conventional bone marrow-derived dendritic cells contribute to toll-like receptor-independent production of alpha/beta interferon in response to inactivated parapoxvirus ovis.
Siegemund S, Hartl A, von Buttlar H, Dautel F, Raue R, Freudenberg MA, Fejer G, Büttner M, Köhler G, Kirschning CJ, Sparwasser T, Alber G
Journal of virology 2009 Sep;83(18):9411-22
Journal of virology 2009 Sep;83(18):9411-22
IL-4Ralpha responsiveness of non-CD4 T cells contributes to resistance in schistosoma mansoni infection in pan-T cell-specific IL-4Ralpha-deficient mice.
Dewals B, Hoving JC, Leeto M, Marillier RG, Govender U, Cutler AJ, Horsnell WG, Brombacher F
The American journal of pathology 2009 Aug;175(2):706-16
The American journal of pathology 2009 Aug;175(2):706-16
Murine neutrophils present Class II restricted antigen.
Culshaw S, Millington OR, Brewer JM, McInnes IB
Immunology letters 2008 Jun 15;118(1):49-54
Immunology letters 2008 Jun 15;118(1):49-54
Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine.
Chen H, Ordög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM
Physiological genomics 2007 Nov 14;31(3):492-509
Physiological genomics 2007 Nov 14;31(3):492-509
Postburn monocytes are the major producers of TNF-alpha in the heterogeneous splenic macrophage population.
Noel G, Guo X, Wang Q, Schwemberger S, Byrum D, Ogle C
Shock (Augusta, Ga.) 2007 Mar;27(3):312-9
Shock (Augusta, Ga.) 2007 Mar;27(3):312-9
Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine.
Chen H, Redelman D, Ro S, Ward SM, Ordög T, Sanders KM
American journal of physiology. Cell physiology 2007 Jan;292(1):C497-507
American journal of physiology. Cell physiology 2007 Jan;292(1):C497-507
Beta cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice.
de Jersey J, Snelgrove SL, Palmer SE, Teteris SA, Mullbacher A, Miller JF, Slattery RM
Proceedings of the National Academy of Sciences of the United States of America 2007 Jan 23;104(4):1295-300
Proceedings of the National Academy of Sciences of the United States of America 2007 Jan 23;104(4):1295-300
CD40/CD154 interactions are required for the optimal maturation of skin-derived APCs and the induction of helminth-specific IFN-gamma but not IL-4.
Hewitson JP, Jenkins GR, Hamblin PA, Mountford AP
Journal of immunology (Baltimore, Md. : 1950) 2006 Sep 1;177(5):3209-17
Journal of immunology (Baltimore, Md. : 1950) 2006 Sep 1;177(5):3209-17
Downregulation of mitogen-activated protein kinases by the Bordetella bronchiseptica Type III secretion system leads to attenuated nonclassical macrophage activation.
Reissinger A, Skinner JA, Yuk MH
Infection and immunity 2005 Jan;73(1):308-16
Infection and immunity 2005 Jan;73(1):308-16
Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly.
Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL
American journal of respiratory cell and molecular biology 2005 Feb;32(2):108-17
American journal of respiratory cell and molecular biology 2005 Feb;32(2):108-17
Viral induction of central nervous system innate immune responses.
Rempel JD, Quina LA, Blakely-Gonzales PK, Buchmeier MJ, Gruol DL
Journal of virology 2005 Apr;79(7):4369-81
Journal of virology 2005 Apr;79(7):4369-81
Helminth infection protects mice from anaphylaxis via IL-10-producing B cells.
Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG
Journal of immunology (Baltimore, Md. : 1950) 2004 Nov 15;173(10):6346-56
Journal of immunology (Baltimore, Md. : 1950) 2004 Nov 15;173(10):6346-56
Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice.
Zheng SJ, Jiang J, Shen H, Chen YH
Journal of immunology (Baltimore, Md. : 1950) 2004 Nov 1;173(9):5652-8
Journal of immunology (Baltimore, Md. : 1950) 2004 Nov 1;173(9):5652-8
Protection against progressive leishmaniasis by IFN-beta.
Mattner J, Wandersee-Steinhäuser A, Pahl A, Röllinghoff M, Majeau GR, Hochman PS, Bogdan C
Journal of immunology (Baltimore, Md. : 1950) 2004 Jun 15;172(12):7574-82
Journal of immunology (Baltimore, Md. : 1950) 2004 Jun 15;172(12):7574-82
Lung cell responses to M. tuberculosis in genetically susceptible and resistant mice following intratracheal challenge.
Eruslanov EB, Majorov KB, Orlova MO, Mischenko VV, Kondratieva TK, Apt AS, Lyadova IV
Clinical and experimental immunology 2004 Jan;135(1):19-28
Clinical and experimental immunology 2004 Jan;135(1):19-28
Control of Leishmania major in the absence of Tyk2 kinase.
Schleicher U, Mattner J, Blos M, Schindler H, Röllinghoff M, Karaghiosoff M, Müller M, Werner-Felmayer G, Bogdan C
European journal of immunology 2004 Feb;34(2):519-29
European journal of immunology 2004 Feb;34(2):519-29
Quantitative analysis by flow cytometry of interstitial cells of Cajal, pacemakers, and mediators of neurotransmission in the gastrointestinal tract.
Ordög T, Redelman D, Horváth VJ, Miller LJ, Horowitz B, Sanders KM
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2004 Dec;62(2):139-49
Cytometry. Part A : the journal of the International Society for Analytical Cytology 2004 Dec;62(2):139-49
Mycobacterial dissemination and cellular responses after 1-lobe restricted tuberculosis infection of genetically susceptible and resistant mice.
Mischenko VV, Kapina MA, Eruslanov EB, Kondratieva EV, Lyadova IV, Young DB, Apt AS
The Journal of infectious diseases 2004 Dec 15;190(12):2137-45
The Journal of infectious diseases 2004 Dec 15;190(12):2137-45
The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response.
Cook AD, Braine EL, Hamilton JA
Journal of immunology (Baltimore, Md. : 1950) 2003 Nov 1;171(9):4816-23
Journal of immunology (Baltimore, Md. : 1950) 2003 Nov 1;171(9):4816-23
Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase.
Blos M, Schleicher U, Soares Rocha FJ, Meissner U, Röllinghoff M, Bogdan C
European journal of immunology 2003 May;33(5):1224-34
European journal of immunology 2003 May;33(5):1224-34
A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection.
Reading PC, Smith GL
The Journal of general virology 2003 Aug;84(Pt 8):1973-1983
The Journal of general virology 2003 Aug;84(Pt 8):1973-1983
In vitro and in vivo regulation by macrophage migration inhibitory factor (MIF) of expression of MHC-II, costimulatory, adhesion, receptor, and cytokine molecules.
Stavitsky AB, Xianli J
Cellular immunology 2002 May-Jun;217(1-2):95-104
Cellular immunology 2002 May-Jun;217(1-2):95-104
Tumor-infiltrating macrophages induce apoptosis in activated CD8(+) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide.
Saio M, Radoja S, Marino M, Frey AB
Journal of immunology (Baltimore, Md. : 1950) 2001 Nov 15;167(10):5583-93
Journal of immunology (Baltimore, Md. : 1950) 2001 Nov 15;167(10):5583-93
CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ.
Radoja S, Saio M, Frey AB
Journal of immunology (Baltimore, Md. : 1950) 2001 May 15;166(10):6074-83
Journal of immunology (Baltimore, Md. : 1950) 2001 May 15;166(10):6074-83
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
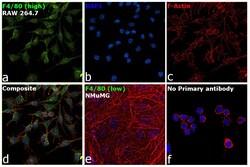
- Experimental details
- Immunofluorescence analysis of F4/80 was performed using 70% confluent log phase RAW 264.7 and NMuMG cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with F4/80 Monoclonal Antibody (CI:A3-1) (Product # MA1-91124) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then with Goat anti-Rat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Product # A-11006) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic and membrane localization. Panel e represents NMuMG cells having no expression of F4/80. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
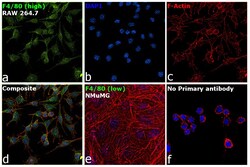
- Experimental details
- Immunofluorescence analysis of F4/80 was performed using 70% confluent log phase RAW 264.7 and NMuMG cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with F4/80 Monoclonal Antibody (CI:A3-1) (Product # MA1-91124) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then with Goat anti-Rat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Product # A-11006) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic and membrane localization. Panel e represents NMuMG cells having no expression of F4/80. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
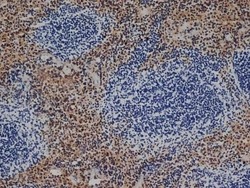
- Experimental details
- Immunohistochemistry analysis of F4/80 was performed in mouse spleen tissue using F4/80 Monoclonal Antibody (CI:A3-1) (Product # MA1-91124).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
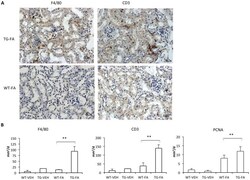
- Figure 7 FA injection induces interstitial cell infiltration in transgenic line A homozygous mice. The inflammatory cell infiltration was characterized by immunohistochemistry with anti-F4/80 (monocytes/macrophages) and anti-CD3 (T cells) antibodies. (A and B). Representative immunostaining of one mouse from each group (x400 magnification). (C) Quantification of positive IHC signals were quantified for (a) F4/80, (b) CD3 and (c) PCNA using KS300 image analyzer software. All parameters were significantly increased in transgenic mice. Data are shown as the mean +- SEM of 14-18 mice per group * p < 0.05; ** p < 0.01 vs WT-FA.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 1 miR-23a is upregulated in DRG neurons derived from mice after SNI. ( A ) miR-23a expression in contralateral L5 DRG neurons at 7 day after model establishment, normalized to U6 (Scale bar = 50 mum) and quantitative histogram of miR-23a + neurons using FISH; ( B ) miR-23a expression in DRG neurons of sham-operated and SNI mice were determined using RT-qPCR, normalized to U6; ( C ) macrophages in DRG (F4/80+ cells, red) and miR-23a (green) detected using FISH and immunohistochemistry (Scale bar = 50 mum); ( D ) miR-23a expression in DRG neurons treated with 0.001% DMSO in HEPES buffer + glucose (1 mg/mL) and with capsaicin (1 muM), normalized to U6. Values obtained from three independent experiments are expressed as mean +- SD and analyzed by unpaired t -test. * indicates p < 0.05. Cell experiment was independently repeated for three times.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 6 Delivery of decreased miR-23a by DRG neuron-derived EVs reduces neuropathic hypersensitivity and recruitment of M1 macrophages in vivo . SNI mice were treated with intrathecal administration of EVs-treated miR-23a antagomir-transfected DRG neurons. ( A ) effect of intrathecal injection of EVs-miR-23a antagomir on mechanical hypersensitivity for 7 days, which was expressed as mean +- SD of 50% PWT; n (vehicle mice) = 6, n (oligomer-treated mice) = 12; * p < 0.05 vs. vehicle mice; # p < 0.05 vs. oligomer-treated mice. Values obtained from three independent experiments in triplicate are expressed as mean +- SD and analyzed by two-way ANOVA, followed by Tukey''s test among multiple groups. ( B ) miR-23a expression in DRG determined using RT-qPCR, normalized to U6; ( C ) Immunostaining of macrophages (F4/80+ cells, red), and nuclei (DAPI, blue) in SNI DRG (scale bar = 25 mum); ( D ) Macrophage phenotyping [M1 macrophages (CD206 - CD11c + ) and M2 macrophages (CD206 + CD11c - )] in DRG analyzed using flow cytometry; ( E ) levels of TNF-alpha and IL-6 in L4-L5 DRG tissues measured using ELISA. Values obtained from three independent experiments are expressed as mean +- SD and analyzed by unpaired t -test between two groups in panel ( B - E ). * p < 0.05 vs. mice treated with EVs-miR-23a-NC or vehicle. Cell experiment was independently repeated for three times.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
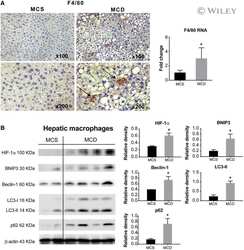
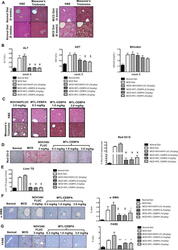
- Fig. 5 The effects of MTL-CEBPA on MCD-induced fatty liver disease. a Histological analysis of liver sections from normal diet and MCD diet-fed animals for ( a , upper panels) 4 weeks and ( a , lower panels) 6 weeks stained with H&E and Masson's trichome (magnification: x 20). ( a , left panel) Analysis of liver sections from normal diet control animals showed normal architecture with hepatocytes arranged in the form of cords (blue arrow) radiating away from the central vein. The portal trip (red arrow) contained an artery, vein, and the bile duct. Normal distribution of the sinusoidal spaces (yellow arrow) was observed. ( a , right panel) Analysis of the liver sections from MCD diet animals revealed steatosis (green arrow) and inflammatory cell infiltration (black arrow). b Serum levels of ALT and AST indicate reduction to near normal levels following 2 weeks of MTL-CEBPA treatment. Bilirubin levels do not continue to increase in the presence of 1 and 3 mg/kg of MTL-CEBPA treatment. Y= = p (0.0002); * = p (0.002); $ = p (0.003). Histological analysis of liver sections from MCD diet-fed animals treated with NOV340/FLUC (3 mg/kg) and MTL-CEBPA at 0.3 mg/kg; 1.0 mg/kg and 3 mg/kg. c Liver sections stained with H&E and Masson's trichrome. (magnification: x 20). Analysis of liver sections from MCD + NOV340/FLUC revealed steatosis (green arrow) and inflammatory cell infiltration (black arrow) which was more prominent, when compared with MCD diet at week 6. The liver sections from
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
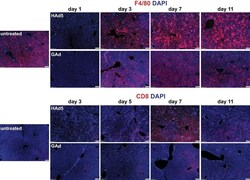
- FIG 5 Rapid kinetics of liver Kupffer cell depletion followed by low levels of inflammatory cell infiltration to liver in mice following intravenous injection of GAd. Immunofluorescence microscopy analysis of F4/80 + macrophages (top two rows) and CD8 + T cells (bottom two rows) in liver from untreated C57BL/6J mice (untreated, n = 3) and from mice at days 1, 3, 5, 7, and 11 following intravenous injection of 1.0 x 10 11 viral particles (vp) of HAd5 and GAd ( n = 2 for each virus and time point). Magnifications, x100. Red, CD31/endomucin; green, GFP; blue, DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
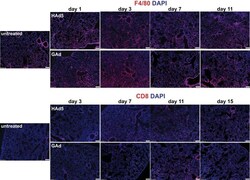
- FIG 7 Lack of substantial inflammatory cell infiltration into lung in mice following intravenous injection of GAd. Immunofluorescence microscopy was used to analyze F4/80 + macrophages and CD8 + T cells in lungs from untreated C57BL/6J mice (untreated, n = 3) and from mice at days 1, 3, 7, and 11 following intravenous injection of 1.0 x 10 11 viral particles (vp) of HAd5 and GAd ( n = 2 for each virus and time point). Magnifications, x100. Red, CD31/endomucin; green, GFP; blue, DAPI.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
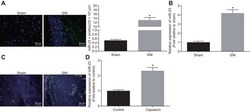
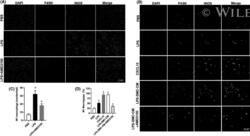
- 5 FIGURE In vivo: AMD3100 inhibited LPS-stimulated M1 macrophage polarization. (A, B) Fluorescence confocal microscopy analysis of M1 macrophage polarization. Representative images of IF showing DAPI, F4/80 +ve , iNOS +ve and merged images. Nuclei were stained by DAPI and are shown in blue. F4/80 marks macrophages while iNOS is a marker of M1 macrophages. (A) Uterus tissue from mice treated with PBS, LPS or LPS + AMD3100 respectively. (B) Macrophages treated with PBS, LPS, CXCL12, LPS-SMC-CM and LPS-SMC-CM + AMD3100 respectively. (C) Quantitative analyses of macrophage polarization (iNOS +ve cells) in uterus showing significant increase in polarization in LPS-treated macrophages compared to PBS. Treatment with AMD3100 inhibited the polarization stimulated by LPS. (D) Quantitative analyses of macrophage polarization (iNOS +ve cells) in vitro showing significant increase in polarization in LPS, CXCL12 and LPS-SMC-CM treated macrophages compared with PBS treatment. AMD3100 inhibited the polarization stimulated by LPS. Results shown as mean +- SE. * p < 0.05 versus PBS while # p < 0.05 versus LPS-SMC-CM. Scale bar: 75 mum. DAPI, 4,6-diamidino-2-phenylindole; IF, immunofluorescence; IHC, immunohistochemistry; LPS, lipopolysaccharide; PBS, phosphate buffered saline; SE, standard error; SMC-CM, smooth muscle cell conditioned media
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
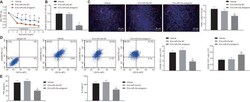
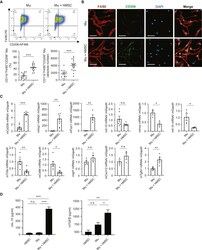
- Increased M2Mph by co-culture with hAM-MSCs in vitro Mouse bone marrow-derived Mph were co-cultured with hAM-MSCs using a Transwell insert (0.4 mum pore size) for 48 h to investigate the role of hAM-MSC secretome on Mph activation. (A) Representative flow cytometry dot plots of Mph with or without co-culture with hAM-MSCs (Mph + hMSC and Mph groups, respectively). Cells double positive for F4/80 and CD206 within the CD11b + population were defined as M2Mph. Percentage of M2Mph among the total live cells as well as the absolute number of M2Mph was calculated and presented in the charts. (B) Immunofluorescent images for F4/80, CD206, and DAPI of Mph, demonstrating enhanced CD206 expression by co-culture with hAM-MSCs compared to the ordinary Mph monoculture. Scale bars, 20 mum. (C) RT-PCR quantification of Mph gene expression after hAM-MSC co-culture (Mph + hMSC group) compared to the Mph-only group using GAPDH as an internal reference gene. Fold change in mRNA levels was normalized to 1 for the control group. n = 4 biological replicates with 3 technical replicates. (D) Secretion levels of IL-10 and TGF-beta1 of Mph with or without co-culture with hAM-MSCs. n = 4 biological replicates with 3 technical replicates. Data are shown as mean +- SEM. *p < 0.05, **p < 0.01, and ***p < 0.001. Student's t test (A and C); one-way ANOVA with Tukey's post hoc tests (D).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
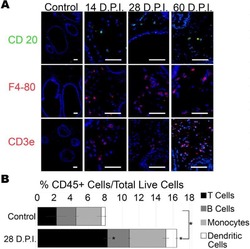
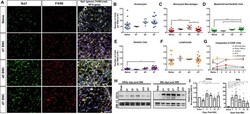
- Figure 2. Immune cell profiles in axotomized DRGs. ( A ) Representative images of L4 DRG cross sections from naive mice, d1, d3, and d7 post-SNC. Macrophages were stained with anti-Iba1 and anti-F4/80. Neurons were stained with anti-NFH. Scale bar, 50 um. ( B ) Quantification of granulocytes per DRG detected by flow cytometry. For flow cytometry of DRGs, naive mice (n = 14 biological replicates), d1 (n = 3), d3 (n = 5), and d7 (n = 12) post-SNC mice were used. Granulocytes (CD45 + , CD11b + , Ly6G + ) per DRG are shown. ( C ) Quantification of Mo/Mac (CD45 + , CD11b + , CD11c - , Ly6G - ) per DRG. ( D ) Quantification of MoDC (CD45 + , CD11b + , CD11c + , Ly6G - ) per DRG. ( E ) Quantification of cDC (CD45 + , CD11b - , CD11c + , Ly6G - ) per DRG. ( F ) Quantification of lymphocytes (CD45+, CD11b - ) per DRG. ( G ) Composition of CD45 + leukocytes in lumbar DRGs identified by flow cytometry. Flow data are represented as mean cell number +- SEM. Each data point represents L3-L5 DRGs pooled from three to four animals (18-24 DRGs), biological replicates, n = 3-14. Statistical analysis was performed in GraphPad Prism (v7) using one-way or two-way ANOVA with correction for multiple comparisons with Tukey's post-hoc test. For B-F, unpaired two-tailed t-test with Welch's correction. A p value < 0.05 (*) was considered significant. p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
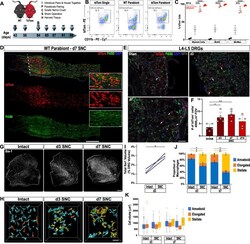
- Figure 3. Sciatic nerve injury triggers massive accumulation of hematogenous leukocytes in the injured nerve but not axotomized DRGs. ( A ) Parbiosis complex of a wildtype (WT) and a tdTomato (tdTom) mouse. Mice were surgically paired at postnatal day 56. The timeline of the experiment is shown. ( B ) Flow cytometric analysis of sciatic nerve trunks collected from non-parabiotic (single) tdTom mice, WT parabionts, and tdTom parabionts. Dotplot of live (CD11b + , tdTom + ) cells in the d3 post-SNC nerve. ( C ) Quantification of tdTom + myeloid cells in the 3d injured nerve of WT single mice (WT-S), WT parabiont (WT-para), tdTom parabiont (tdTom-para), and tdTom single (tdTom-S) mice. The fraction of tdTom + myeloid cells (CD45 + , CD11b + ), MoDC (CD45 + , CD11b + , CD11c + , Ly6G - ), and Mo/Mac (CD45 + , CD11b + , CD11c - , Ly6G - ) is shown. For quantification of tdTom + immune cells, nerves from the WT parabiont and the tdTom parabiont were harvested separately (three mice per data point) with n = 2-3 biological replicates. Flow data are represented as fraction of tdTom + cells +- SEM. Statistical analysis was performed in GraphPad Prism (v8) using one-way ANOVA with correction for multiple comparisons with Tukey's post-hoc test. p value of < 0.001 (***) and p
 Explore
Explore Validate
Validate Learn
Learn