33-1700
antibody from Invitrogen Antibodies
Targeting: PLK1
PLK
 Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [41]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [2]
- Flow cytometry [1]
- Other assay [32]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 33-1700 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PLK1 Antibody Cocktail
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- 33-1700 contains a cocktail of monoclonal mouse PLK1 containing clone PL6, isotype IgG1, kappa and clone PY20, isotype IgG2b, kappa.
- Reactivity
- Human, Mouse, Rat, Xenopus
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- PL6/PL2
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Linking post-translational modifications and protein turnover by site-resolved protein turnover profiling.
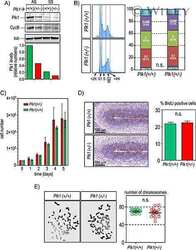
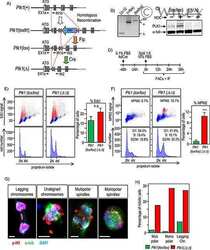
Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival.
Development of a Novel Cell-Permeable Protein-Protein Interaction Inhibitor for the Polo-box Domain of Polo-like Kinase 1.
A kinase-independent function for AURORA-A in replisome assembly during DNA replication initiation.
Preferential Killing of Tetraploid Colon Cancer Cells by Targeting the Mitotic Kinase PLK1.
HSP70 is required for the proper assembly of pericentriolar material and function of mitotic centrosomes.
A cryptic hydrophobic pocket in the polo-box domain of the polo-like kinase PLK1 regulates substrate recognition and mitotic chromosome segregation.
Clathrin heavy chain phosphorylated at T606 plays a role in proper cell division.
CDC20B is required for deuterosome-mediated centriole production in multiciliated cells.
Biochemical analyses reveal amino acid residues critical for cell cycle-dependent phosphorylation of human Cdc14A phosphatase by cyclin-dependent kinase 1.
A PIM-CHK1 signaling pathway regulates PLK1 phosphorylation and function during mitosis.
Plk1 overexpression induces chromosomal instability and suppresses tumor development.
A Class of Environmental and Endogenous Toxins Induces BRCA2 Haploinsufficiency and Genome Instability.
Modulating Protein-Protein Interactions of the Mitotic Polo-like Kinases to Target Mutant KRAS.
Hypoxia-induced alterations of G2 checkpoint regulators.
Genetic depletion of Polo-like kinase 1 leads to embryonic lethality due to mitotic aberrancies.
TOPBP1 regulates RAD51 phosphorylation and chromatin loading and determines PARP inhibitor sensitivity.
USP9X stabilizes XIAP to regulate mitotic cell death and chemoresistance in aggressive B-cell lymphoma.
High-resolution Antibody Array Analysis of Childhood Acute Leukemia Cells.
Pharmacological inhibition of Polo-like kinase 1 (PLK1) by BI-2536 decreases the viability and survival of hamartin and tuberin deficient cells via induction of apoptosis and attenuation of autophagy.
PLK1 regulation of PCNT cleavage ensures fidelity of centriole separation during mitotic exit.
SNW1 enables sister chromatid cohesion by mediating the splicing of sororin and APC2 pre-mRNAs.
NFBD1/MDC1 is phosphorylated by PLK1 and controls G2/M transition through the regulation of a TOPOIIα-mediated decatenation checkpoint.
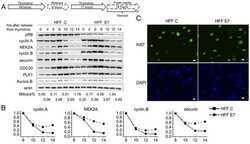
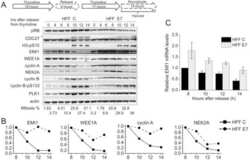
Human papillomavirus type 16 E7 oncoprotein inhibits the anaphase promoting complex/cyclosome activity by dysregulating EMI1 expression in mitosis.
Human cells enter mitosis with damaged DNA after treatment with pharmacological concentrations of genotoxic agents.
Human Cdc14A regulates Wee1 stability by counteracting CDK-mediated phosphorylation.
CHFR protein regulates mitotic checkpoint by targeting PARP-1 protein for ubiquitination and degradation.
Phosphorylation of Ran-binding protein-1 by Polo-like kinase-1 is required for interaction with Ran and early mitotic progression.
Substrate degradation by the anaphase promoting complex occurs during mitotic slippage.
SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of JNK inhibition.
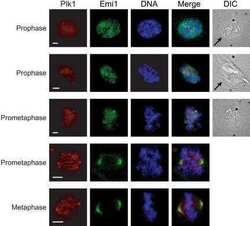
DNA damage triggers p21WAF1-dependent Emi1 down-regulation that maintains G2 arrest.
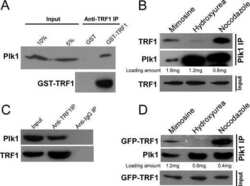
Plk1 phosphorylation of TRF1 is essential for its binding to telomeres.
p73-mediated transcriptional activity is negatively regulated by polo-like kinase 1.
CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit.
Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress.
Radiosensitization of colorectal carcinoma cell lines by histone deacetylase inhibition.
Response of malignant B lymphocytes to ionizing radiation: gene expression and genotype.
p53-regulated transcriptional program associated with genotoxic stress-induced apoptosis.
Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC Inhibitor Emi1.
Mitotic regulation of the human anaphase-promoting complex by phosphorylation.
The G(2) DNA damage checkpoint delays expression of genes encoding mitotic regulators.
Zecha J, Gabriel W, Spallek R, Chang YC, Mergner J, Wilhelm M, Bassermann F, Kuster B
Nature communications 2022 Jan 10;13(1):165
Nature communications 2022 Jan 10;13(1):165
Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival.
Dietachmayr M, Rathakrishnan A, Karpiuk O, von Zweydorf F, Engleitner T, Fernández-Sáiz V, Schenk P, Ueffing M, Rad R, Eilers M, Gloeckner CJ, Clemm von Hohenberg K, Bassermann F
Nature communications 2020 Mar 9;11(1):1268
Nature communications 2020 Mar 9;11(1):1268
Development of a Novel Cell-Permeable Protein-Protein Interaction Inhibitor for the Polo-box Domain of Polo-like Kinase 1.
Huggins DJ, Hardwick BS, Sharma P, Emery A, Laraia L, Zhang F, Narvaez AJ, Roberts-Thomson M, Crooks AT, Boyle RG, Boyce R, Walker DW, Mateu N, McKenzie GJ, Spring DR, Venkitaraman AR
ACS omega 2020 Jan 14;5(1):822-831
ACS omega 2020 Jan 14;5(1):822-831
A kinase-independent function for AURORA-A in replisome assembly during DNA replication initiation.
Guarino Almeida E, Renaudin X, Venkitaraman AR
Nucleic acids research 2020 Aug 20;48(14):7844-7855
Nucleic acids research 2020 Aug 20;48(14):7844-7855
Preferential Killing of Tetraploid Colon Cancer Cells by Targeting the Mitotic Kinase PLK1.
Jemaà M, Kifagi C, Serrano SS, Massoumi R
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2020 Apr 8;54(2):303-320
Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2020 Apr 8;54(2):303-320
HSP70 is required for the proper assembly of pericentriolar material and function of mitotic centrosomes.
Fang CT, Kuo HH, Hsu SC, Yih LH
Cell division 2019;14:4
Cell division 2019;14:4
A cryptic hydrophobic pocket in the polo-box domain of the polo-like kinase PLK1 regulates substrate recognition and mitotic chromosome segregation.
Sharma P, Mahen R, Rossmann M, Stokes JE, Hardwick B, Huggins DJ, Emery A, Kunciw DL, Hyvönen M, Spring DR, McKenzie GJ, Venkitaraman AR
Scientific reports 2019 Nov 4;9(1):15930
Scientific reports 2019 Nov 4;9(1):15930
Clathrin heavy chain phosphorylated at T606 plays a role in proper cell division.
Yabuno Y, Uchihashi T, Sasakura T, Shimizu H, Naito Y, Fukushima K, Ota K, Kogo M, Nojima H, Yabuta N
Cell cycle (Georgetown, Tex.) 2019 Aug;18(16):1976-1994
Cell cycle (Georgetown, Tex.) 2019 Aug;18(16):1976-1994
CDC20B is required for deuterosome-mediated centriole production in multiciliated cells.
Revinski DR, Zaragosi LE, Boutin C, Ruiz-Garcia S, Deprez M, Thomé V, Rosnet O, Gay AS, Mercey O, Paquet A, Pons N, Ponzio G, Marcet B, Kodjabachian L, Barbry P
Nature communications 2018 Nov 7;9(1):4668
Nature communications 2018 Nov 7;9(1):4668
Biochemical analyses reveal amino acid residues critical for cell cycle-dependent phosphorylation of human Cdc14A phosphatase by cyclin-dependent kinase 1.
Ovejero S, Ayala P, Malumbres M, Pimentel-Muiños FX, Bueno A, Sacristán MP
Scientific reports 2018 Aug 8;8(1):11871
Scientific reports 2018 Aug 8;8(1):11871
A PIM-CHK1 signaling pathway regulates PLK1 phosphorylation and function during mitosis.
Adam K, Cartel M, Lambert M, David L, Yuan L, Besson A, Mayeux P, Manenti S, Didier C
Journal of cell science 2018 Aug 10;131(15)
Journal of cell science 2018 Aug 10;131(15)
Plk1 overexpression induces chromosomal instability and suppresses tumor development.
de Cárcer G, Venkateswaran SV, Salgueiro L, El Bakkali A, Somogyi K, Rowald K, Montañés P, Sanclemente M, Escobar B, de Martino A, McGranahan N, Malumbres M, Sotillo R
Nature communications 2018 Aug 1;9(1):3012
Nature communications 2018 Aug 1;9(1):3012
A Class of Environmental and Endogenous Toxins Induces BRCA2 Haploinsufficiency and Genome Instability.
Tan SLW, Chadha S, Liu Y, Gabasova E, Perera D, Ahmed K, Constantinou S, Renaudin X, Lee M, Aebersold R, Venkitaraman AR
Cell 2017 Jun 1;169(6):1105-1118.e15
Cell 2017 Jun 1;169(6):1105-1118.e15
Modulating Protein-Protein Interactions of the Mitotic Polo-like Kinases to Target Mutant KRAS.
Narvaez AJ, Ber S, Crooks A, Emery A, Hardwick B, Guarino Almeida E, Huggins DJ, Perera D, Roberts-Thomson M, Azzarelli R, Hood FE, Prior IA, Walker DW, Boyce R, Boyle RG, Barker SP, Torrance CJ, McKenzie GJ, Venkitaraman AR
Cell chemical biology 2017 Aug 17;24(8):1017-1028.e7
Cell chemical biology 2017 Aug 17;24(8):1017-1028.e7
Hypoxia-induced alterations of G2 checkpoint regulators.
Hasvold G, Lund-Andersen C, Lando M, Patzke S, Hauge S, Suo Z, Lyng H, Syljuåsen RG
Molecular oncology 2016 May;10(5):764-73
Molecular oncology 2016 May;10(5):764-73
Genetic depletion of Polo-like kinase 1 leads to embryonic lethality due to mitotic aberrancies.
Wachowicz P, Fernández-Miranda G, Marugán C, Escobar B, de Cárcer G
BioEssays : news and reviews in molecular, cellular and developmental biology 2016 Jul;38 Suppl 1:S96-S106
BioEssays : news and reviews in molecular, cellular and developmental biology 2016 Jul;38 Suppl 1:S96-S106
TOPBP1 regulates RAD51 phosphorylation and chromatin loading and determines PARP inhibitor sensitivity.
Moudry P, Watanabe K, Wolanin KM, Bartkova J, Wassing IE, Watanabe S, Strauss R, Troelsgaard Pedersen R, Oestergaard VH, Lisby M, Andújar-Sánchez M, Maya-Mendoza A, Esashi F, Lukas J, Bartek J
The Journal of cell biology 2016 Feb 1;212(3):281-8
The Journal of cell biology 2016 Feb 1;212(3):281-8
USP9X stabilizes XIAP to regulate mitotic cell death and chemoresistance in aggressive B-cell lymphoma.
Engel K, Rudelius M, Slawska J, Jacobs L, Ahangarian Abhari B, Altmann B, Kurutz J, Rathakrishnan A, Fernández-Sáiz V, Brunner A, Targosz BS, Loewecke F, Gloeckner CJ, Ueffing M, Fulda S, Pfreundschuh M, Trümper L, Klapper W, Keller U, Jost PJ, Rosenwald A, Peschel C, Bassermann F
EMBO molecular medicine 2016 Aug;8(8):851-62
EMBO molecular medicine 2016 Aug;8(8):851-62
High-resolution Antibody Array Analysis of Childhood Acute Leukemia Cells.
Kanderova V, Kuzilkova D, Stuchly J, Vaskova M, Brdicka T, Fiser K, Hrusak O, Lund-Johansen F, Kalina T
Molecular & cellular proteomics : MCP 2016 Apr;15(4):1246-61
Molecular & cellular proteomics : MCP 2016 Apr;15(4):1246-61
Pharmacological inhibition of Polo-like kinase 1 (PLK1) by BI-2536 decreases the viability and survival of hamartin and tuberin deficient cells via induction of apoptosis and attenuation of autophagy.
Valianou M, Cox AM, Pichette B, Hartley S, Paladhi UR, Astrinidis A
Cell cycle (Georgetown, Tex.) 2015;14(3):399-407
Cell cycle (Georgetown, Tex.) 2015;14(3):399-407
PLK1 regulation of PCNT cleavage ensures fidelity of centriole separation during mitotic exit.
Kim J, Lee K, Rhee K
Nature communications 2015 Dec 9;6:10076
Nature communications 2015 Dec 9;6:10076
SNW1 enables sister chromatid cohesion by mediating the splicing of sororin and APC2 pre-mRNAs.
van der Lelij P, Stocsits RR, Ladurner R, Petzold G, Kreidl E, Koch B, Schmitz J, Neumann B, Ellenberg J, Peters JM
The EMBO journal 2014 Nov 18;33(22):2643-58
The EMBO journal 2014 Nov 18;33(22):2643-58
NFBD1/MDC1 is phosphorylated by PLK1 and controls G2/M transition through the regulation of a TOPOIIα-mediated decatenation checkpoint.
Ando K, Ozaki T, Hirota T, Nakagawara A
PloS one 2013;8(12):e82744
PloS one 2013;8(12):e82744
Human papillomavirus type 16 E7 oncoprotein inhibits the anaphase promoting complex/cyclosome activity by dysregulating EMI1 expression in mitosis.
Yu Y, Munger K
Virology 2013 Nov;446(1-2):251-9
Virology 2013 Nov;446(1-2):251-9
Human cells enter mitosis with damaged DNA after treatment with pharmacological concentrations of genotoxic agents.
Kubara PM, Kernéis-Golsteyn S, Studény A, Lanser BB, Meijer L, Golsteyn RM
The Biochemical journal 2012 Sep 15;446(3):373-81
The Biochemical journal 2012 Sep 15;446(3):373-81
Human Cdc14A regulates Wee1 stability by counteracting CDK-mediated phosphorylation.
Ovejero S, Ayala P, Bueno A, Sacristán MP
Molecular biology of the cell 2012 Dec;23(23):4515-25
Molecular biology of the cell 2012 Dec;23(23):4515-25
CHFR protein regulates mitotic checkpoint by targeting PARP-1 protein for ubiquitination and degradation.
Kashima L, Idogawa M, Mita H, Shitashige M, Yamada T, Ogi K, Suzuki H, Toyota M, Ariga H, Sasaki Y, Tokino T
The Journal of biological chemistry 2012 Apr 13;287(16):12975-84
The Journal of biological chemistry 2012 Apr 13;287(16):12975-84
Phosphorylation of Ran-binding protein-1 by Polo-like kinase-1 is required for interaction with Ran and early mitotic progression.
Hwang HI, Ji JH, Jang YJ
The Journal of biological chemistry 2011 Sep 23;286(38):33012-20
The Journal of biological chemistry 2011 Sep 23;286(38):33012-20
Substrate degradation by the anaphase promoting complex occurs during mitotic slippage.
Lee J, Kim JA, Margolis RL, Fotedar R
Cell cycle (Georgetown, Tex.) 2010 May;9(9):1792-801
Cell cycle (Georgetown, Tex.) 2010 May;9(9):1792-801
SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of JNK inhibition.
Kim JA, Lee J, Margolis RL, Fotedar R
Oncogene 2010 Mar 18;29(11):1702-16
Oncogene 2010 Mar 18;29(11):1702-16
DNA damage triggers p21WAF1-dependent Emi1 down-regulation that maintains G2 arrest.
Lee J, Kim JA, Barbier V, Fotedar A, Fotedar R
Molecular biology of the cell 2009 Apr;20(7):1891-902
Molecular biology of the cell 2009 Apr;20(7):1891-902
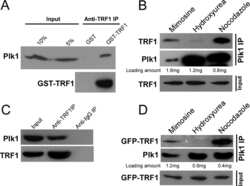
Plk1 phosphorylation of TRF1 is essential for its binding to telomeres.
Wu ZQ, Yang X, Weber G, Liu X
The Journal of biological chemistry 2008 Sep 12;283(37):25503-25513
The Journal of biological chemistry 2008 Sep 12;283(37):25503-25513
p73-mediated transcriptional activity is negatively regulated by polo-like kinase 1.
Soond SM, Barry SP, Melino G, Knight RA, Latchman DS, Stephanou A
Cell cycle (Georgetown, Tex.) 2008 May 1;7(9):1214-23
Cell cycle (Georgetown, Tex.) 2008 May 1;7(9):1214-23
CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit.
Hansen DV, Tung JJ, Jackson PK
Proceedings of the National Academy of Sciences of the United States of America 2006 Jan 17;103(3):608-13
Proceedings of the National Academy of Sciences of the United States of America 2006 Jan 17;103(3):608-13
Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress.
Mailand N, Bekker-Jensen S, Bartek J, Lukas J
Molecular cell 2006 Aug 4;23(3):307-18
Molecular cell 2006 Aug 4;23(3):307-18
Radiosensitization of colorectal carcinoma cell lines by histone deacetylase inhibition.
Flatmark K, Nome RV, Folkvord S, Bratland A, Rasmussen H, Ellefsen MS, Fodstad Ø, Ree AH
Radiation oncology (London, England) 2006 Aug 3;1:25
Radiation oncology (London, England) 2006 Aug 3;1:25
Response of malignant B lymphocytes to ionizing radiation: gene expression and genotype.
Lyng H, Landsverk KS, Kristiansen E, DeAngelis PM, Ree AH, Myklebost O, Hovig E, Stokke T
International journal of cancer 2005 Jul 20;115(6):935-42
International journal of cancer 2005 Jul 20;115(6):935-42
p53-regulated transcriptional program associated with genotoxic stress-induced apoptosis.
Kho PS, Wang Z, Zhuang L, Li Y, Chew JL, Ng HH, Liu ET, Yu Q
The Journal of biological chemistry 2004 May 14;279(20):21183-92
The Journal of biological chemistry 2004 May 14;279(20):21183-92
Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC Inhibitor Emi1.
Hansen DV, Loktev AV, Ban KH, Jackson PK
Molecular biology of the cell 2004 Dec;15(12):5623-34
Molecular biology of the cell 2004 Dec;15(12):5623-34
Mitotic regulation of the human anaphase-promoting complex by phosphorylation.
Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM
The EMBO journal 2003 Dec 15;22(24):6598-609
The EMBO journal 2003 Dec 15;22(24):6598-609
The G(2) DNA damage checkpoint delays expression of genes encoding mitotic regulators.
Crawford DF, Piwnica-Worms H
The Journal of biological chemistry 2001 Oct 5;276(40):37166-77
The Journal of biological chemistry 2001 Oct 5;276(40):37166-77
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
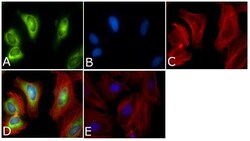
- Experimental details
- Immunofluorescent analysis of PLK1 Antibody (PL6/PL2) was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with PLK1 Antibody (PL6/PL2) (Product # 33-1700) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cytoplasmic and Nuclear localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
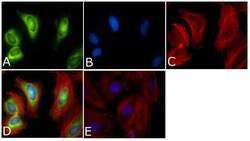
- Experimental details
- Immunofluorescent analysis of PLK1 Antibody (PL6/PL2) was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with PLK1 Antibody (PL6/PL2) (Product # 33-1700) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor 488 Rabbit Anti-Mouse IgG Secondary Antibody (Product # A-11059) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (Product # A12381). Panel d is a merged image showing cytoplasmic and Nuclear localization. Panel e is a no primary antibody control. The images were captured at 40X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
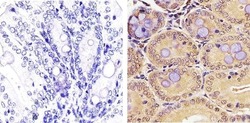
- Experimental details
- Immunohistochemistry analysis of PLK1 showing staining in the nucleus and cytoplasm of paraffin-embedded mouse colon tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a PLK1 monoclonal antibody (Product # 33-1700) diluted in 3% BSA-PBS at a dilution of 1:50 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
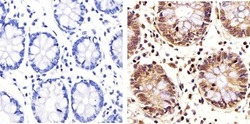
- Experimental details
- Immunohistochemistry analysis of PLK1 showing staining in the nucleus and cytoplasm of paraffin-embedded human colon tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a PLK1 monoclonal antibody (Product # 33-1700) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
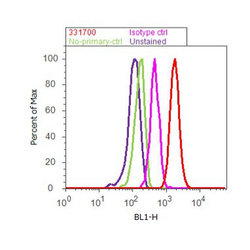
- Experimental details
- Flow cytometry analysis of PLK1 was done on K562 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with PLK1 Mouse Monoclonal Antibody (331700, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
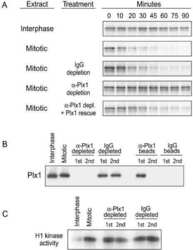
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
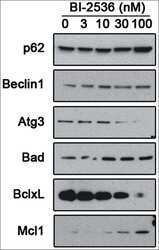
- Figure 5. Pharmacological inhibition of PLK1 alters protein levels of autophagy andBcl - 2 family member proteins. 621-101 cells were treated for24 hours with the indicated concentrations of BI-2536, and the lysates wereimmunoblotted with the indicated antibodies.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
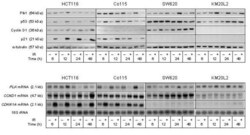
- Figure 2 Cell cycle regulatory factors following exposure to ionizing radiation (IR) . Four colorectal carcinoma cell lines (HCT116, Co115, SW620, and KM20L2) were exposed (+) to 8 Gy of IR, or left unexposed (-), and further incubated for the indicated time periods before analysis. Upper panel: Protein expression levels of Plk1, p53, Cyclin D1, and p21 were analyzed by Western blot immunostaining, using alpha-tubulin as protein loading control. Lower panel: mRNA expression levels of PLK , CCND1 , and CDKN1A were analyzed by Northern blot hybridization, using 18S rRNA as RNA loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
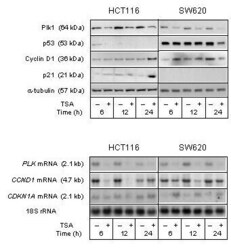
- Figure 5 Cell cycle regulatory factors upon TSA treatment . The HCT116 and SW620 cell lines were treated (+) with 100 nM TSA, or left untreated (-), and further incubated for the indicated time periods before analysis. Upper panel: Protein expression levels of Plk1, p53, Cyclin D1, and p21 were analyzed by Western blot immunostaining, using alpha-tubulin as protein loading control. Lower panel: mRNA expression levels of PLK , CCND1 , and CDKN1A were analyzed by Northern blot hybridization, using 18S rRNA as RNA loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
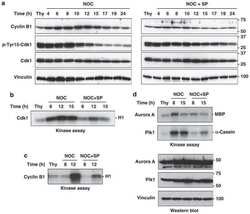
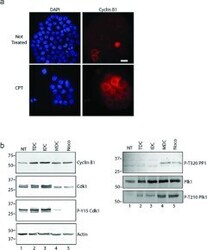
- Figure 3 MDCs express cyclin B1 and the active form of Cdk1 ( a ) Cells were grown on coverslips and either not treated (upper row) or treated with CPT for 48 h (lower row), stained with DAPI (blue) and with anti-(cyclin B1) antibodies (red). Cells were analysed by immunofluorescence microscopy. Scale bar, 50 mum. ( b ) Extracts were prepared from cells either not treated (NT) or treated with CPT. CPT-treated cells were analysed further as total cultures (TDC) or cultures fractionated by mechanical shake-off into adherent interphase cells (IDC) or rounded cells (MDC). Extracts were also prepared from cells treated with nocodazole (Noco). Samples were processed by Western blotting with antibodies against cyclin B1, Cdk1, phospho-Tyr 15 Cdk1 (P-Y15 Cdk1), phospho-Thr 320 PP1a (P-T320 PP1), Plk1, phospho-Thr 210 Plk1 (P-T210 Plk1) or actin. Molecular masses are indicated in kDa.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
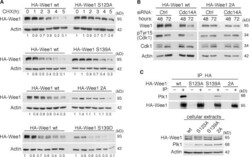
- FIGURE 7: (A) Phosphorylation of Wee1 at Ser-139 is required for normal Wee1 turnover. HEK293T cells were transfected with HA-tagged, wild-type Wee1 (wt) or the different HA-tagged Wee1 mutants (S123A, S139A, S139D, and S123/139A or 2A). At 24 h posttransfection, cells were treated with cycloheximide to inhibit protein synthesis and then harvested at the indicated time points to check HA-Wee1 protein levels by immunoblotting. HA-Wee1 bands were quantified and normalized with respect to actin. HA-Wee1 protein levels from 0 h were considered as 1. The data are representative of three independent experiments. (B) Cdc14A dephosphorylates Wee1 at Ser-123 and Ser-139 to regulate Wee1 stability. U-2-OS cells stably expressing retrovirally transduced, HA-tagged Wee1 wt or the double 2A mutant were treated with control or Cdc14A siRNAs for 48 or 72 h. Cellular extracts were obtained and analyzed by immunoblotting against the indicated proteins. These blots are representative of three independent experiments. (C) Recombinant HA-Wee1 proteins (wt, S123A, S139A, or 2A mutants) were expressed in HEK293T cells, immunoprecipitated with anti-HA antibodies, and analyzed by immunoblotting with the indicated antibodies. This experiment was performed twice.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
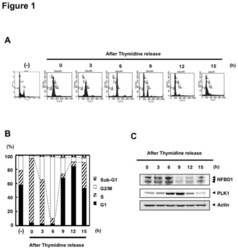
- Experimental details
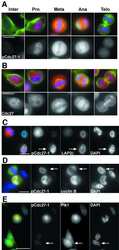
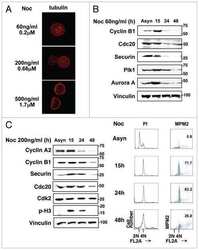
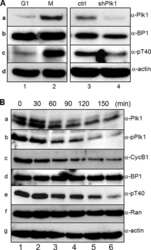
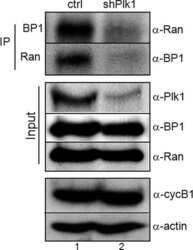
- Figure 1 NFBD1 and PLK1 are both expressed in the G2/M phase and form a protein complex. (A) HeLa cells were double-thymidine blocked at the late G1 phase and then released into fresh medium. At the indicated times after release, cells were stained with propidium iodide (PI) and analyzed by FACS. (B) Schematic representation of cell-cycle distributions under the abovementioned experimental conditions. (C) Western blot analysis. At the indicated time after release, whole cell lysates were prepared and immunoblotted against anti-NFBD1 (first panel), anti-PLK1 (second panel), or anti-actin (third panel) antibody. Immunoblotting for actin is shown as a control for protein loading.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
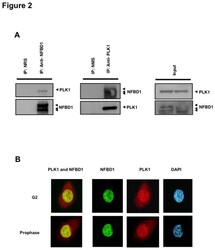
- Experimental details
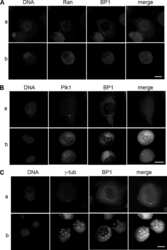
- Figure 2 Co-immunoprecipitation and co-localization between NFBD1 and PLK1 in the G2/M phase. (A) Immunoprecipitation analysis. HeLa cells were synchronized by the double-thymidine block regimen. Six hours after the second release, whole cell lysates prepared from HeLa cells were immunoprecipitated with normal rabbit serum or with a polyclonal anti-NFBD1 antibody followed by immunoblotting with a monoclonal anti-PLK1 antibody (left panel). Reciprocal experiments using normal mouse serum and a monoclonal anti-PLK1 antibody are shown in the middle panel. (B) Indirect immunofluorescence staining. HeLa cells were synchronized by the double-thymidine block regimen. Six hours after the second release, mitotic cells were simultaneously stained with polyclonal anti-NFBD1 (green) and monoclonal anti-PLK1 antibodies (red). Merged images (yellow) indicate the co-localization of NFBD1 with PLK1. Nuclear DNA was stained with DAPI (blue).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
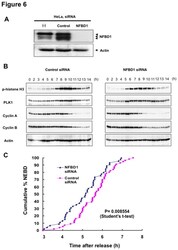
- Experimental details
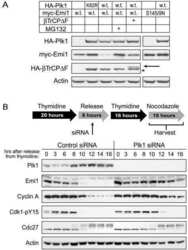
- Figure 6 Knockdown of NFBD1 accelerates G2/M progression. (A) siRNA-mediated knockdown of NFBD1 results in a reduction of endogenous NFBD1. HeLa cells were synchronized by the double-thymidine block regimen and transfected with 10 nM of NFBD1 siRNA or control siRNA at the first release. At the time of the second release, whole cell lysates prepared from transfected cells were subjected to immunoblotting with anti-NFBD1 antibody (top panel). Western blotting for actin is shown as a control for protein loading (bottom panel). (B) Western blotting analysis of mitosis-related proteins. Whole cell lysates were prepared in NFBD1 siRNA transfected cells (right panel) or control siRNA transfected cells (left panel) at the indicated times after the second release and immunoblotted with anti-phospho-histone H3 antibody, anti-PLK1 antibody, anti-cyclin A antibody, anti-cyclin B antibody, or anti-actin antibody. (C) Cumulative percentages of NEBD-positive cells. GFP-cyclin B-expressing HeLa cells were synchronized and transfected with the indicated siRNAs. The times of NEBD were defined by the loss of a defined nuclear boundary and nuclear accumulation of cyclin B in the NFBD1 siRNA-transfected cells (n= 45) and control siRNA-transfected cells (n= 56). The significant difference was demonstrated by the Student's t -test.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
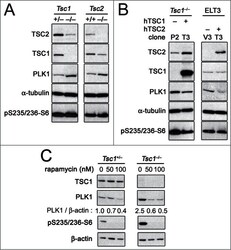
- Experimental details
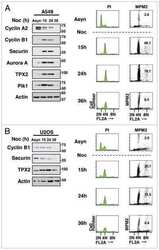
- Figure 1. PLK1 protein levels are increased in hamartin and tuberin deficient cells and arerapamycin-sensitive. ( A ) Lysates from exponentially growing Tsc1 +/- , Tsc1 -/- , Tsc2 +/+ / Tp53 -/- and Tsc2 -/- / Tp53 -/- MEF were immunoblotted with the indicated antibodies. ( B ) Lysates from Tsc1 -/- MEF transduced with vector(208-P2) or TSC1 (208-T3), and from tuberin-deficient ELT3 cells transduced withvector (ELT3-V3) or TSC2 (ELT3-T3) were immunoblotted with the indicated antibodies.( C ) Lysates from Tsc1 +/- and Tsc1 -/- MEF treated for 24 hours with0, 50 or 100 nM rapamycin were immunoblotted with the indicated antibodies.PLK1 protein levels normalized to beta-actin and to vehicle-treated Tsc1 +/- MEF are indicated below the PLK1immunoblots.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
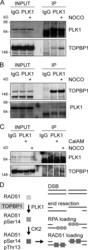
- Experimental details
- Figure 5. TOPBP1 interacts with PLK1. Immunoblots of control, anti-PLK1 (A) or anti-TOPBP1 (B) immunoprecipitates (IPs) from U2OS cells treated or not with nocodazole (40 ng/ml) for 4 h before collection. (C) Immunoblots of control and anti-PLK1 IPs from U2OS cells treated or not with CalAM (4 uM) or nocodazole (40 ng/ml) for 4 h before collection. (D) Model of TOPBP1 role in HR.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
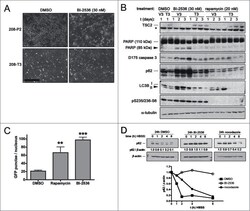
- Experimental details
- Figure 4. Pharmacological inhibition of PLK1 induces apoptosis in hamartin and tuberindeficient cells and attenuates autophagy. ( A ) Phase contrastmicrographs of hamartin deficient 208-P2 MEF and hamartin re-expressing 208-T3 MEFtreated with vehicle control (DMSO) or 30 nM BI-2536 for 3 days. Scale bar400 mum. ( B ) Tuberin deficient ELT3-V3 and tuberin re-expressingELT3-T3 cells were treated with vehicle control (DMSO), 30 nM BI-2536, or20 nM rapamycin for 1, 2 and 3 days, and lysates were immunoblotted withthe indicated antibodies. Asterisk (*) on tuberin immunoblot indicates anon-specific band identified on rat species with the anti-tuberin antibody used, andwas previously described. 50 ( C ) WI38 primary fibroblasts ectopically expressing GFP-LC3 weretreated with vehicle control (DMSO), 20 nM rapamycin, or 100 nM BI-2536for 24 hours, fixed, and the number of GFP-LC3 punctae per nucleus wasquantified from epi-fluorescence digital micrographs. n >= 9,** and *** indicate P < 0.01 and P < 0.001, respectively, compared to DMSO. ( D )HEK293 cells were pre-treated for 24 hours with 0.1% v/v DMSO, or100 nM BI-2536, or 70 ng/ml nocodazole, media were removed, cellmonolayers were washed twice with HBSS, incubated for the indicated time with HBSSin the presence of pre-treatment compounds, lysed, and lysates were immunoblottedfor p62 and beta-actin. The p62 / beta-actin ratios (normalized for the 0 htime-point within each treatment group) are shown below the p62 immunoblots and atth
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
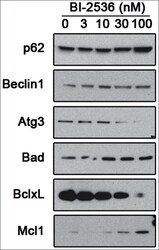
- Experimental details
- Figure 5. Pharmacological inhibition of PLK1 alters protein levels of autophagy andBcl - 2 family member proteins. 621-101 cells were treated for24 hours with the indicated concentrations of BI-2536, and the lysates wereimmunoblotted with the indicated antibodies.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
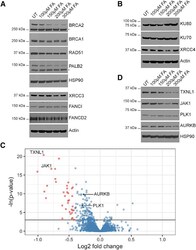
- Experimental details
- Figure 4 Formaldehyde Selectively Depletes Components of the Cellular Proteome (A) Abundance of proteins involved in homologous recombination or the Fanconi anemia repair pathway in wild-type HeLa Kyoto cells treated with FA for 5 hr. (B) Abundance of proteins involved in non-homologous end-joining in HeLa Kyoto cells treated with FA for 5 hr. (C) Volcano plot showing the results of the SWATH-MS analysis of HeLa Kyoto cells treated with or without 200 muM FA for 5 hr. Each dot represents a protein with Benjamini-Hochberg adjusted p values plotted along the y axis, and the fold change in abundance following FA treatment along the x axis. The horizontal black line indicates where p = 0.05. Red dots mark proteins that are depleted by >=25% compared to untreated controls in a statistically significant manner (p < 0.05). Proteins tested by western blotting are labeled. (D) Abundance of selected proteins from SWATH-MS analysis in HeLa Kyoto cells treated with FA for 5 hr. See also Figure S4 and Tables S1 and S2 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
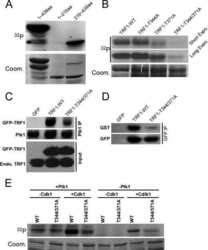
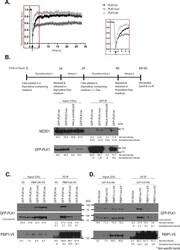
- Figure 4 Differential engagement of PLK1 PBD substrates via the Tyr pocket. ( A ) FRAP analysis of interphase centrosomes in live cells. GFP-PLK1 Wt/AAD/AM at the centrosome was photobleached and its recovery monitored at 0.2 s intervals. The median t 1/2 time taken for 50% maximal recovery for GFP-PLK1 Wt/AAD/AM were found to be 0.7 s, 0.4 s and 0.3 s respectively. The arrowhead indicates the point of photobleaching. An inset shows data points for the first 5 seconds including photobleaching and recovery time of GFP signal. ( B ) HeLa cells expressing GFP-PLK1 Wt/AAD/AM were synchronized in mitosis by double thymidine block and released as shown in the experimental schedule. The cell lysates were immunoprecipitated using GFP-Trap(r) beads to pull down GFP-PLK1 Wt/AAD/AM and analysed by immunoblotting. The data presented is representative of two independent experiments. ( C ) HeLa cells expressing GFP-PLK1 Wt/AAD/AM were transfected with PBIP1 Wt -V5 or pcDNA(tm)3.1/V5-HisA vector (or V5 vector) and harvested 24 h later. PBIP1 Wt -V5/ V5 was pulled down from the lysates using V5 antibody and immunoprecipitates were analysed by immunoblotting. Asterisks (*) indicate cross-reacting bands. The data presented is representative of two independent experiments. ( D ) HeLa GFP-PLK1 Wt overexpressing cells were transfected with either PBIP1 Wt/F71A/T78A -V5 or V5 vector and harvested 24 h later. PBIP1 Wt/F71A/T78A -V5 was pulled down from the lysates using V5 antibody and analysed by
 Explore
Explore Validate
Validate Learn
Learn