Antibody data
- Antibody Data
- Antigen structure
- References [26]
- Comments [0]
- Validations
- Immunohistochemistry [1]
- Other assay [16]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 459210 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- NDUFB8 Monoclonal Antibody (20E9DH10C12)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Positive control: Isolated mitochondria from Human heart, bovine heart, rat heart and mouse heart. Skeletal muscle tissue.
- Reactivity
- Human, Mouse, Rat, Bovine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 20E9DH10C12
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- 4°C
Submitted references Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2.
Raman Spectroscopic Assessment of Myocardial Viability in Langendorff-Perfused Ischemic Rat Hearts.
PPARδ Attenuates Alcohol-Mediated Insulin Resistance by Enhancing Fatty Acid-Induced Mitochondrial Uncoupling and Antioxidant Defense in Skeletal Muscle.
Mitochondrial Deoxyguanosine Kinase Regulates NAD(+) Biogenesis Independent of Mitochondria Complex I Activity.
GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function.
A novel approach to measure mitochondrial respiration in frozen biological samples.
Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men.
Chloramphenicol Mitigates Oxidative Stress by Inhibiting Translation of Mitochondrial Complex I in Dopaminergic Neurons of Toxin-Induced Parkinson's Disease Model.
Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments.
Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice.
The mitochondrial deoxyguanosine kinase is required for cancer cell stemness in lung adenocarcinoma.
AIF promotes a JNK1-mediated cadherin switch independently of respiratory chain stabilization.
Mild Impairment of Mitochondrial OXPHOS Promotes Fatty Acid Utilization in POMC Neurons and Improves Glucose Homeostasis in Obesity.
APP promotes osteoblast survival and bone formation by regulating mitochondrial function and preventing oxidative stress.
Perturbations of NAD(+) salvage systems impact mitochondrial function and energy homeostasis in mouse myoblasts and intact skeletal muscle.
Increased mitophagy in the skeletal muscle of spinal and bulbar muscular atrophy patients.
The mitochondrial translation machinery as a therapeutic target in Myc-driven lymphomas.
Basal metabolic state governs AIF-dependent growth support in pancreatic cancer cells.
AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD.
Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin.
Activation of sirtuin 1 as therapy for the peroxisomal disease adrenoleukodystrophy.
Targeting FoxO1 with AS1842856 suppresses adipogenesis.
Independent roles of methionine sulfoxide reductase A in mitochondrial ATP synthesis and as antioxidant in retinal pigment epithelial cells.
Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy.
The enzymatic activity of apoptosis-inducing factor supports energy metabolism benefiting the growth and invasiveness of advanced prostate cancer cells.
Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice.
Chella Krishnan K, Vergnes L, Acín-Pérez R, Stiles L, Shum M, Ma L, Mouisel E, Pan C, Moore TM, Péterfy M, Romanoski CE, Reue K, Björkegren JLM, Laakso M, Liesa M, Lusis AJ
Nature metabolism 2021 Nov;3(11):1552-1568
Nature metabolism 2021 Nov;3(11):1552-1568
Raman Spectroscopic Assessment of Myocardial Viability in Langendorff-Perfused Ischemic Rat Hearts.
Ikemoto K, Hashimoto K, Harada Y, Kumamoto Y, Hayakawa M, Mochizuki K, Matsuo K, Yashiro K, Yaku H, Takamatsu T, Tanaka H
Acta histochemica et cytochemica 2021 Apr 28;54(2):65-72
Acta histochemica et cytochemica 2021 Apr 28;54(2):65-72
PPARδ Attenuates Alcohol-Mediated Insulin Resistance by Enhancing Fatty Acid-Induced Mitochondrial Uncoupling and Antioxidant Defense in Skeletal Muscle.
Koh JH, Kim KH, Park SY, Kim YW, Kim JY
Frontiers in physiology 2020;11:749
Frontiers in physiology 2020;11:749
Mitochondrial Deoxyguanosine Kinase Regulates NAD(+) Biogenesis Independent of Mitochondria Complex I Activity.
Sang L, He YJ, Kang J, Ye H, Bai W, Luo XD, Sun J
Frontiers in oncology 2020;10:570656
Frontiers in oncology 2020;10:570656
GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function.
Timper K, Del Río-Martín A, Cremer AL, Bremser S, Alber J, Giavalisco P, Varela L, Heilinger C, Nolte H, Trifunovic A, Horvath TL, Kloppenburg P, Backes H, Brüning JC
Cell metabolism 2020 Jun 2;31(6):1189-1205.e13
Cell metabolism 2020 Jun 2;31(6):1189-1205.e13
A novel approach to measure mitochondrial respiration in frozen biological samples.
Acin-Perez R, Benador IY, Petcherski A, Veliova M, Benavides GA, Lagarrigue S, Caudal A, Vergnes L, Murphy AN, Karamanlidis G, Tian R, Reue K, Wanagat J, Sacks H, Amati F, Darley-Usmar VM, Liesa M, Divakaruni AS, Stiles L, Shirihai OS
The EMBO journal 2020 Jul 1;39(13):e104073
The EMBO journal 2020 Jul 1;39(13):e104073
Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men.
Dollerup OL, Chubanava S, Agerholm M, Søndergård SD, Altıntaş A, Møller AB, Høyer KF, Ringgaard S, Stødkilde-Jørgensen H, Lavery GG, Barrès R, Larsen S, Prats C, Jessen N, Treebak JT
The Journal of physiology 2020 Feb;598(4):731-754
The Journal of physiology 2020 Feb;598(4):731-754
Chloramphenicol Mitigates Oxidative Stress by Inhibiting Translation of Mitochondrial Complex I in Dopaminergic Neurons of Toxin-Induced Parkinson's Disease Model.
Han J, Kim SJ, Ryu MJ, Jang Y, Lee MJ, Ju X, Lee YL, Cui J, Shong M, Heo JY, Kweon GR
Oxidative medicine and cellular longevity 2019;2019:4174803
Oxidative medicine and cellular longevity 2019;2019:4174803
Fascin Controls Metastatic Colonization and Mitochondrial Oxidative Phosphorylation by Remodeling Mitochondrial Actin Filaments.
Lin S, Huang C, Gunda V, Sun J, Chellappan SP, Li Z, Izumi V, Fang B, Koomen J, Singh PK, Hao J, Yang S
Cell reports 2019 Sep 10;28(11):2824-2836.e8
Cell reports 2019 Sep 10;28(11):2824-2836.e8
Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice.
Polyzos AA, Lee DY, Datta R, Hauser M, Budworth H, Holt A, Mihalik S, Goldschmidt P, Frankel K, Trego K, Bennett MJ, Vockley J, Xu K, Gratton E, McMurray CT
Cell metabolism 2019 Jun 4;29(6):1258-1273.e11
Cell metabolism 2019 Jun 4;29(6):1258-1273.e11
The mitochondrial deoxyguanosine kinase is required for cancer cell stemness in lung adenocarcinoma.
Lin S, Huang C, Sun J, Bollt O, Wang X, Martine E, Kang J, Taylor MD, Fang B, Singh PK, Koomen J, Hao J, Yang S
EMBO molecular medicine 2019 Dec;11(12):e10849
EMBO molecular medicine 2019 Dec;11(12):e10849
AIF promotes a JNK1-mediated cadherin switch independently of respiratory chain stabilization.
Scott AJ, Walker SA, Krank JJ, Wilkinson AS, Johnson KM, Lewis EM, Wilkinson JC
The Journal of biological chemistry 2018 Sep 21;293(38):14707-14722
The Journal of biological chemistry 2018 Sep 21;293(38):14707-14722
Mild Impairment of Mitochondrial OXPHOS Promotes Fatty Acid Utilization in POMC Neurons and Improves Glucose Homeostasis in Obesity.
Timper K, Paeger L, Sánchez-Lasheras C, Varela L, Jais A, Nolte H, Vogt MC, Hausen AC, Heilinger C, Evers N, Pospisilik JA, Penninger JM, Taylor EB, Horvath TL, Kloppenburg P, Brüning JC
Cell reports 2018 Oct 9;25(2):383-397.e10
Cell reports 2018 Oct 9;25(2):383-397.e10
APP promotes osteoblast survival and bone formation by regulating mitochondrial function and preventing oxidative stress.
Pan JX, Tang F, Xiong F, Xiong L, Zeng P, Wang B, Zhao K, Guo H, Shun C, Xia WF, Mei L, Xiong WC
Cell death & disease 2018 Oct 22;9(11):1077
Cell death & disease 2018 Oct 22;9(11):1077
Perturbations of NAD(+) salvage systems impact mitochondrial function and energy homeostasis in mouse myoblasts and intact skeletal muscle.
Agerholm M, Dall M, Jensen BAH, Prats C, Madsen S, Basse AL, Graae AS, Risis S, Goldenbaum J, Quistorff B, Larsen S, Vienberg SG, Treebak JT
American journal of physiology. Endocrinology and metabolism 2018 Apr 1;314(4):E377-E395
American journal of physiology. Endocrinology and metabolism 2018 Apr 1;314(4):E377-E395
Increased mitophagy in the skeletal muscle of spinal and bulbar muscular atrophy patients.
Borgia D, Malena A, Spinazzi M, Desbats MA, Salviati L, Russell AP, Miotto G, Tosatto L, Pegoraro E, Sorarù G, Pennuto M, Vergani L
Human molecular genetics 2017 Mar 15;26(6):1087-1103
Human molecular genetics 2017 Mar 15;26(6):1087-1103
The mitochondrial translation machinery as a therapeutic target in Myc-driven lymphomas.
D'Andrea A, Gritti I, Nicoli P, Giorgio M, Doni M, Conti A, Bianchi V, Casoli L, Sabò A, Mironov A, Beznoussenko GV, Amati B
Oncotarget 2016 Nov 8;7(45):72415-72430
Oncotarget 2016 Nov 8;7(45):72415-72430
Basal metabolic state governs AIF-dependent growth support in pancreatic cancer cells.
Scott AJ, Wilkinson AS, Wilkinson JC
BMC cancer 2016 Apr 23;16:286
BMC cancer 2016 Apr 23;16:286
AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD.
Brandauer J, Andersen MA, Kellezi H, Risis S, Frøsig C, Vienberg SG, Treebak JT
Frontiers in physiology 2015;6:85
Frontiers in physiology 2015;6:85
Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin.
Braun M, Hettinger N, Koentges C, Pfeil K, Cimolai MC, Hoffmann MM, Osterholt M, Doenst T, Bode C, Bugger H
PloS one 2015;10(3):e0119416
PloS one 2015;10(3):e0119416
Activation of sirtuin 1 as therapy for the peroxisomal disease adrenoleukodystrophy.
Morató L, Ruiz M, Boada J, Calingasan NY, Galino J, Guilera C, Jové M, Naudí A, Ferrer I, Pamplona R, Serrano M, Portero-Otín M, Beal MF, Fourcade S, Pujol A
Cell death and differentiation 2015 Nov;22(11):1742-53
Cell death and differentiation 2015 Nov;22(11):1742-53
Targeting FoxO1 with AS1842856 suppresses adipogenesis.
Zou P, Liu L, Zheng L, Liu L, Stoneman RE, Cho A, Emery A, Gilbert ER, Cheng Z
Cell cycle (Georgetown, Tex.) 2014;13(23):3759-67
Cell cycle (Georgetown, Tex.) 2014;13(23):3759-67
Independent roles of methionine sulfoxide reductase A in mitochondrial ATP synthesis and as antioxidant in retinal pigment epithelial cells.
Dun Y, Vargas J, Brot N, Finnemann SC
Free radical biology & medicine 2013 Dec;65:1340-1351
Free radical biology & medicine 2013 Dec;65:1340-1351
Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy.
Morató L, Galino J, Ruiz M, Calingasan NY, Starkov AA, Dumont M, Naudí A, Martínez JJ, Aubourg P, Portero-Otín M, Pamplona R, Galea E, Beal MF, Ferrer I, Fourcade S, Pujol A
Brain : a journal of neurology 2013 Aug;136(Pt 8):2432-43
Brain : a journal of neurology 2013 Aug;136(Pt 8):2432-43
The enzymatic activity of apoptosis-inducing factor supports energy metabolism benefiting the growth and invasiveness of advanced prostate cancer cells.
Lewis EM, Wilkinson AS, Jackson JS, Mehra R, Varambally S, Chinnaiyan AM, Wilkinson JC
The Journal of biological chemistry 2012 Dec 21;287(52):43862-75
The Journal of biological chemistry 2012 Dec 21;287(52):43862-75
Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice.
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R
Molecular neurodegeneration 2011 Jun 6;6:39
Molecular neurodegeneration 2011 Jun 6;6:39
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
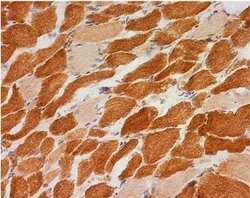
- Experimental details
- Immunohistochemical analysis of NDUFB8 in skeletal muscle using an NDUFB8 Monoclonal antibody (Product # 459210). Samples are frozen skeletal muscle tissue sections from a patient with a single large deletion of the mtDNA. The image shows a mosaic of complex I positive and complex I negative fibers.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2 The mitochondrial translation machinery regulates global mitochondrial activity and Emu -myc cell growth A. Emu- myc lymphoma cells were transduced with shRNAs against Ptcd3 , Mrps5 , Mrps27 , Renilla luciferase (Ren) or a control empty vector (EV). The number of viable cells at each time point (top) was determined by trypan blue staining. For each single shRNA the efficiency of knockdown was evaluated by real-time qPCR three days post-transduction (bottom). Transcript abundance is expressed as mean +- s.d. of triplicate measurements, expressed relative to the EV control and normalized to Rplp0 . B. Doubling time was calculated from the growth curves in A., using the formula described in experimental procedures. C. Cell death was evaluated by Annexin V and propidium iodide (PI) staining at the 48hrs time-point. The graph shows the percentage of Annexin V+/PI- (black) and Annexin V+/PI+ cells (grey) corresponding to early and late apoptotic cells, respectively. D. Western blot analysis of components of the Electron Transport Chain (ETC) Complexes I-IV following 48hrs of knock-down. As loading controls, we used antibodies against cytoplasmic Vinculin and the mitochondrial Voltage-dependent anion channel (VDAC). E. Real-time qPCR quantification of mtDNA, normalized to nuclear DNA. Results are shown as mean +- s.d. from triplicate measurements. F. Oxygen consumption rate (OCR) was determined as described in experimental procedures at 48hrs. Data were normalized to total
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
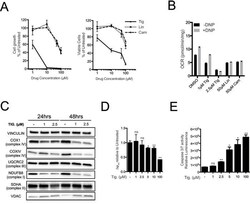
- Figure 3 Tigecycline impairs the growth of Emu -myc lymphoma cells and inhibits translation of mitochondrial proteins A. Dose-response curve of Emu- myc lymphoma cells treated with the indicated doses of Tigecycline (Tig), Linezolid (Lin) or Cloramphenicol (Cam) for 48hrs. Data are shown as cell numbers (left panel) or viability (right panel), as percentage of untreated cells (mock-treated with DMSO). Cell number and viability was determined by trypan blue staining. B. Oxygen consumption rate (OCR) measured as defined in Figure 2F on Emu- myc lymphoma cells treated with the indicated antibiotics or carrier (DMSO). To measure spare respiratory capacity, cells were treated with the ETC uncoupler 2,4-Dinitrophenol (DNP). C. Western blot analysis of components of the ETC complexes I-IV. Cells were treated with the indicated concentration of Tigecycline for 24 or 48hrs. The loading controls are Vinculin and VDAC, as in Figure 2D . D. Mitochondrial membrane potential was determined by staining with the cationic cyanine dye DilC1(5). E. Cleaved caspase 3/7 activity was determined with the caspase 3/7 glo assay luminescent kit. Measurements in D and E were taken after 6 hrs of treatment. Means, s.d. and statistical significance are as defined in Figure 2 . See also Supplementary Figures S3 and S4 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
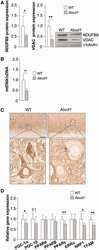
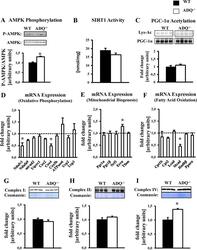
- Fig 5 Preserved OXPHOS protein levels and mitochondrial biogenic signaling in ADQ -/- hearts. AMPK phosphorylation (A), SIRT1 activity (B), lysine acetylation (Lys-Ac) of PGC-1alpha (C), mRNA expression of OXPHOS subunits (D), mRNA expression of mitochondrial biogenesis signaling molecules (E), mRNA expression of fatty acid oxidation genes and PPARalpha (F), and mitochondrial protein levels of OXPHOS complexes I (NDUFB8 subunit; G), II (Fp subunit; H), and IV (subunit IV; I) in hearts of ADQ -/- and WT mice at 8 weeks of age; n = 4-5. Myocardial mRNA expression is expressed relative to WT expression which was set to 1 (indicated by the dotted line). * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
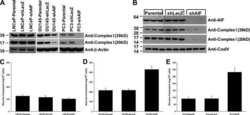
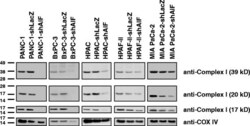
- Fig. 5 AIF selectively controls respiratory chain protein expression in pancreatic cancer cells. Following suppression of AIF, respiratory chain status was assessed by immunoblot analysis of complex I (39-, 20-, and 17-kDa subunits) and COX IV
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
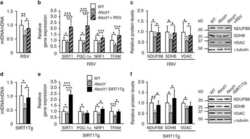
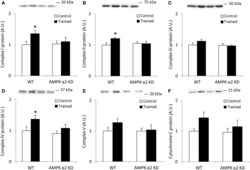
- Figure 1 Exercise training increase mitochondrial oxidative phosphorylation complexes in an AMPK alpha2-dependent manner . Protein levels of oxidative phosphorylation complexes, (A) Complex I, (B) Complex II, (C) Complex III, (D) Complex IV, (E) Complex V (oligomycin-sensitivity conferring protein subunit, OSCP), and (F) Cytochrome C were evaluated in quadriceps muscle of sedentary (control) or exercise trained female WT and AMPK alpha2 KD mice ( n = 13-15). Values are mean +- SEM. * indicates vs. WT control ( p < 0.05) analyzed by unpaired t -tests.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
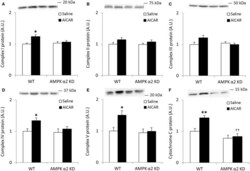
- Figure 4 Repeated treatment with AICAR increases abundance of mitochondrial electron transport chain proteins in an AMPK alpha2-dependent manner . WT and AMPK alpha2 KD male mice were given daily subcutaneous injections with AICAR (500 mg/kg body weight) or saline for 4 weeks. (A-E) Protein abundance of oxidative phosphorylation Complexes I-V and (F) Cytochrome C was measured in quadriceps muscle ( n = 7-8). A significant interaction effect (treatment x genotype; p < 0.05) was observed for Cytochrome C. Values are mean +- SEM. * indicates vs. WT saline ( p < 0.05) analyzed by t -tests, ** indicates vs. WT saline ( p < 0.01), and ++ indicates genotype effect within AICAR treated animals ( p < 0.01).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
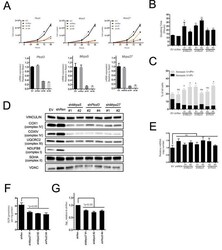
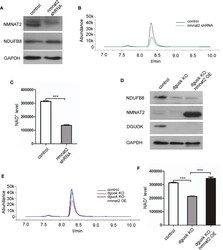
- Figure 3 DGUOK regulates NAD + biosynthesis through NMNAT 2 . (A) Western blot analysis of NMNAT2 and NDUFB8 expression level in NMNAT2 -KD and control H1650 cells. (B) LC-MS analysis of NAD + level in NMNAT2 -KD and control H1650 cells. (C) Quantification of NAD + level in NMNAT2 -KD and control H1650 cells. (D) Western blot analysis of NMNAT2, NDUFB8 and DGUOK expression levels in DGUOK -KO, DGUOK -KO/ NMNAT2 -OE and control H1650 cells. (E) LC-MS analysis of NAD + level in DGUOK -KO, DGUOK -KO/ NMNAT2 -OE and control H1650 cells. (F) Quantification of NAD + level in DGUOK -KO, DGUOK -KO/ NMNAT2 -OE and control H1650 cells. ***p < 0001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
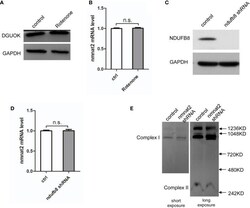
- Figure 4 DGUOK regulates NMNAT2 independent of mitochondria complex I activity. (A) Western blot analysis of DGUOK in control and Rotenone treated H1650 cells. (B) qRT-PCR analysis of the NMNAT2 mRNA level in Rotenone treated and control H1650 cells. (C) Western blot analysis of NDUFB8 in NDUFB8 -KD and control H1650 cells. (D) qRT-PCR analysis of the NMNAT2 mRNA level in NDUFB8 -KD and control H1650 cells. (E) BN-PAGE gel analysis of mitochondria complex I level in NMNAT2 -KD and control H1650 cells. n.s. not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
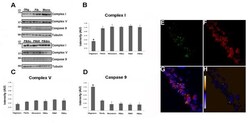
- Figure 6 Tau oligomers induce mitochondrial alterations . (A) Representative western blot of mouse brain homogenate. The levels of complex I, complex V, and caspase-9 were measured by band quantification and normalized with the levels of tubulin. Abbreviations are as in Fig. 4. (B) Complex I levels were significantly lower in the hemisphere injected with tau oligomers in comparison with the ones injected with fibrils or monomers. (C) No differences in the levels of complex V were observed in any of the groups. (D) Caspase-9 activation was significantly higher in tau oligomer groups over both tau fibril- and monomer-injected groups. (E-G) Double staining between human tau-specific antibody HT7 (green fluorescence) and mitochondrial porin antibody (red fluorescence) demonstrates the internalization of tau oligomers in CA1 cells and their interaction with the mitochondria. (H) The co-localization of tau oligomers with mitochondria was confirmed by ICA of the signal. Data are represented as mean +- SE. *p < 0.01, n = 6.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
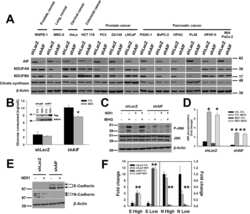
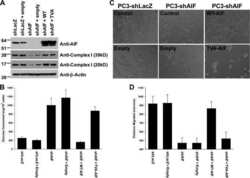
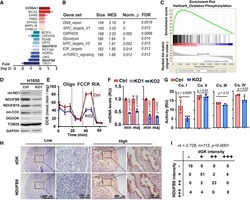
- Figure 3 DGUOK is required for mitochondrial OXPHOS in lung adenocarcinoma cells A Waterfall plot showing fold changes of 17 mitochondrial proteins differentially regulated in DGUOK KO H1650 cells. Fold changes (log 2 ) are presented as mean +- SEM of results from two independent experiments. B Top enriched pathways in DGUOK high lung adenocarcinoma ( n = 100) compared to DGUOK low lung adenocarcinoma ( n = 100) based on Gene Set Enrichment Analysis on RNA-sequencing data from the TCGA database. NES, normalized enrichment score, Norm. p , normalized P value. C Enrichment score profile of the oxidative_phosphorylation pathway in GSEA in (B). D Western blotting showing the decrease in respiration complex I subunits in DGUOK KO H1650 cells. E The effects of DGUOK depletion on mitochondrial oxygen consumption rate in H1650 cells. n = 3 independent replicates per group. F The effects of DGUOK KO on mtDNA copies in H1650 cells. Two pairs of primers recognizing minor Arc (minArc) and major Arc (majArc) region of mtDNA were used for the qPCR quantitation. G The effect of DGUOK depletion on the activities of respiratory complexes I, II, III, and IV. H Representative IHC staining showing the expression of DGUOK and NDUFB8 in lung adenocarcinoma patient specimens. I Correlation between DGUOK staining intensity and NDUFB8 staining intensity in a cohort of 113 lung adenocarcinoma patients. r s = 0.728, P < 0.0001, as determined by Spearman's correlation test. Data information: Data are sh
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
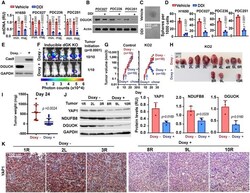
- Figure 7 Genetic targeting of DGUOK inhibits tumor initiation and induces tumor regression A The effect of DDI treatment on mtDNA copies in H1650 cells and three patient-derived cancer cells from lung adenocarcinoma (PDC027, PDC236, and PDC251). Two pairs of primers recognizing minor Arc (minArc) and major Arc (majArc) region of mtDNA were used for the qPCR quantitation. Data are shown as mean +- SD ( n = 3 per group). B The effect of DDI treatment on DGUOK protein levels in the three patient-derived cancer cell lines. C, D Representative images (C) and quantitation data (D) showing the effects of DDI treatment on lung sphere formation in H1650 and patient-derived lung cancer cells. Data are shown as mean +- SD ( n = 4 independent samples per group). E Western blotting showing the doxycycline (doxy)-induced expression of Cas9 and the deletion of DGUOK protein expression after 7 days of treatment. F 1 x 10 5 LLC cells expressing Tet-On Cas9 and DGUOK sgRNA5 (KO2) were inoculated via s.c. into both flanks of Albino BL6 mice. One group of mice ( n = 5 mice, 10 inoculations per group) were provided with doxycycline chow 2 days after inoculation. The formation of tumor was monitored by bioluminescence imaging 28 days postinoculation. P < 0.0001, n = 10 mice per group, Fisher's exact test. G 1 x 10 5 LLC cells stably expressing Tet-On Cas9 and with DGUOK sgRNA (KO2 group) or without sgRNA (control group) were inoculated via s.c. into Albino BL6 mice. Mice were provided with doxycyc
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry