Antibody data
- Antibody Data
- Antigen structure
- References [27]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [15]
- Immunohistochemistry [5]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-016 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- GRP94 Monoclonal Antibody (9G10)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- MA3-016 detects glucose regulated protein 94 kDa (GRP94) from human, rat, mouse, chick, hamster and bovine tissues. MA3-016 is also expected to react with GRP94 from Xenopus, human, canine, rabbit, non-human primate, canine, Drosophila, and ovine tissues. MA3-016 has been successfully used in Western blot, Immunohistochemistry (paraffin), immunocytochemistry, immunofluorescence and immunoprecipitation procedures. By Western blot, this antibody detects an ~108 kDa protein representing GRP94 in chick oviduct cytosol. The MA3-016 immunogen is chick oviduct GRP94.
- Reactivity
- Human, Mouse, Rat, Bovine, Chicken/Avian, Hamster
- Host
- Rat
- Isotype
- IgG
- Antibody clone number
- 9G10
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Cooperative treatment effectiveness of ATR and HSP90 inhibition in Ewing's sarcoma cells.
The inducible β5i proteasome subunit contributes to proinsulin degradation in GRP94-deficient β-cells and is overexpressed in type 2 diabetes pancreatic islets.
Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo.
Cytosolic CD38 protein forms intact disulfides and is active in elevating intracellular cyclic ADP-ribose.
Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death.
Comparative proteomics profiling of a phospholamban mutant mouse model of dilated cardiomyopathy reveals progressive intracellular stress responses.
GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion.
Influence of Ki-ras-driven oncogenic transformation on the protein network of murine fibroblasts.
Relation of the size and intracellular sorting of apoB to the formation of VLDL 1 and VLDL 2.
Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition.
Xenopus as an experimental model for studying evolution of hsp--immune system interactions.
Identification of the N-terminal peptide binding site of glucose-regulated protein 94.
Interaction of neuropeptide Y and Hsp90 through a novel peptide binding region.
Oolemmal proteomics--identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane.
Oolemmal proteomics--identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane.
Glycosylation increases potassium channel stability and surface expression in mammalian cells.
Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca(2+)-ATPase: relationship between global protein synthesis and expression and translation of individual genes.
Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca(2+)-ATPase: relationship between global protein synthesis and expression and translation of individual genes.
Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4.
Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4.
Folding of rabies virus glycoprotein: epitope acquisition and interaction with endoplasmic reticulum chaperones.
Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome.
Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase.
Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase.
hsp108,,,,,, a novel heat shock inducible protein of chicken.
Structural analysis of chicken oviduct progesterone receptor using monoclonal antibodies to the subunit B protein.
Structural analysis of chicken oviduct progesterone receptor using monoclonal antibodies to the subunit B protein.
Marx C, Schaarschmidt MU, Kirkpatrick J, Marx-Blümel L, Halilovic M, Westermann M, Hoelzer D, Meyer FB, Geng Y, Buder K, Schadwinkel HM, Siniuk K, Becker S, Thierbach R, Beck JF, Sonnemann J, Wang ZQ
Cell & bioscience 2021 Mar 20;11(1):57
Cell & bioscience 2021 Mar 20;11(1):57
The inducible β5i proteasome subunit contributes to proinsulin degradation in GRP94-deficient β-cells and is overexpressed in type 2 diabetes pancreatic islets.
Khilji MS, Bresson SE, Verstappen D, Pihl C, Andersen PAK, Agergaard JB, Dahlby T, Bryde TH, Klindt K, Nielsen CK, Walentinsson A, Zivkovic D, Bousquet MP, Tyrberg B, Richardson SJ, Morgan NG, Mandrup-Poulsen T, Marzec MT
American journal of physiology. Endocrinology and metabolism 2020 Jun 1;318(6):E892-E900
American journal of physiology. Endocrinology and metabolism 2020 Jun 1;318(6):E892-E900
Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo.
Elashiry M, Elashiry MM, Elsayed R, Rajendran M, Auersvald C, Zeitoun R, Rashid MH, Ara R, Meghil MM, Liu Y, Arbab AS, Arce RM, Hamrick M, Elsalanty M, Brendan M, Pacholczyk R, Cutler CW
Journal of extracellular vesicles 2020 Aug 7;9(1):1795362
Journal of extracellular vesicles 2020 Aug 7;9(1):1795362
Cytosolic CD38 protein forms intact disulfides and is active in elevating intracellular cyclic ADP-ribose.
Zhao YJ, Zhang HM, Lam CM, Hao Q, Lee HC
The Journal of biological chemistry 2011 Jun 24;286(25):22170-7
The Journal of biological chemistry 2011 Jun 24;286(25):22170-7
Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death.
Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G
The EMBO journal 2009 Mar 4;28(5):578-90
The EMBO journal 2009 Mar 4;28(5):578-90
Comparative proteomics profiling of a phospholamban mutant mouse model of dilated cardiomyopathy reveals progressive intracellular stress responses.
Gramolini AO, Kislinger T, Alikhani-Koopaei R, Fong V, Thompson NJ, Isserlin R, Sharma P, Oudit GY, Trivieri MG, Fagan A, Kannan A, Higgins DG, Huedig H, Hess G, Arab S, Seidman JG, Seidman CE, Frey B, Perry M, Backx PH, Liu PP, MacLennan DH, Emili A
Molecular & cellular proteomics : MCP 2008 Mar;7(3):519-33
Molecular & cellular proteomics : MCP 2008 Mar;7(3):519-33
GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion.
Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y
Molecular biology of the cell 2007 Oct;18(10):3764-75
Molecular biology of the cell 2007 Oct;18(10):3764-75
Influence of Ki-ras-driven oncogenic transformation on the protein network of murine fibroblasts.
Recktenwald CV, Mendler S, Lichtenfels R, Kellner R, Seliger B
Proteomics 2007 Feb;7(3):385-98
Proteomics 2007 Feb;7(3):385-98
Relation of the size and intracellular sorting of apoB to the formation of VLDL 1 and VLDL 2.
Stillemark-Billton P, Beck C, Borén J, Olofsson SO
Journal of lipid research 2005 Jan;46(1):104-14
Journal of lipid research 2005 Jan;46(1):104-14
Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition.
Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ
Journal of cell science 2004 Jul 15;117(Pt 16):3645-57
Journal of cell science 2004 Jul 15;117(Pt 16):3645-57
Xenopus as an experimental model for studying evolution of hsp--immune system interactions.
Robert J, Gantress J, Cohen N, Maniero GD
Methods (San Diego, Calif.) 2004 Jan;32(1):42-53
Methods (San Diego, Calif.) 2004 Jan;32(1):42-53
Identification of the N-terminal peptide binding site of glucose-regulated protein 94.
Gidalevitz T, Biswas C, Ding H, Schneidman-Duhovny D, Wolfson HJ, Stevens F, Radford S, Argon Y
The Journal of biological chemistry 2004 Apr 16;279(16):16543-52
The Journal of biological chemistry 2004 Apr 16;279(16):16543-52
Interaction of neuropeptide Y and Hsp90 through a novel peptide binding region.
Ishiwatari-Hayasaka H, Maruya M, Sreedhar AS, Nemoto TK, Csermely P, Yahara I
Biochemistry 2003 Nov 11;42(44):12972-80
Biochemistry 2003 Nov 11;42(44):12972-80
Oolemmal proteomics--identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane.
Calvert ME, Digilio LC, Herr JC, Coonrod SA
Reproductive biology and endocrinology : RB&E 2003 Feb 14;1:27
Reproductive biology and endocrinology : RB&E 2003 Feb 14;1:27
Oolemmal proteomics--identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane.
Calvert ME, Digilio LC, Herr JC, Coonrod SA
Reproductive biology and endocrinology : RB&E 2003 Feb 14;1:27
Reproductive biology and endocrinology : RB&E 2003 Feb 14;1:27
Glycosylation increases potassium channel stability and surface expression in mammalian cells.
Khanna R, Myers MP, Lainé M, Papazian DM
The Journal of biological chemistry 2001 Sep 7;276(36):34028-34
The Journal of biological chemistry 2001 Sep 7;276(36):34028-34
Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca(2+)-ATPase: relationship between global protein synthesis and expression and translation of individual genes.
Mengesdorf T, Althausen S, Oberndorfer I, Paschen W
The Biochemical journal 2001 Jun 15;356(Pt 3):805-12
The Biochemical journal 2001 Jun 15;356(Pt 3):805-12
Response of neurons to an irreversible inhibition of endoplasmic reticulum Ca(2+)-ATPase: relationship between global protein synthesis and expression and translation of individual genes.
Mengesdorf T, Althausen S, Oberndorfer I, Paschen W
The Biochemical journal 2001 Jun 15;356(Pt 3):805-12
The Biochemical journal 2001 Jun 15;356(Pt 3):805-12
Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4.
Bogan JS, Lodish HF
The Journal of cell biology 1999 Aug 9;146(3):609-20
The Journal of cell biology 1999 Aug 9;146(3):609-20
Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4.
Bogan JS, Lodish HF
The Journal of cell biology 1999 Aug 9;146(3):609-20
The Journal of cell biology 1999 Aug 9;146(3):609-20
Folding of rabies virus glycoprotein: epitope acquisition and interaction with endoplasmic reticulum chaperones.
Gaudin Y
Journal of virology 1997 May;71(5):3742-50
Journal of virology 1997 May;71(5):3742-50
Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome.
Hughes EA, Hammond C, Cresswell P
Proceedings of the National Academy of Sciences of the United States of America 1997 Mar 4;94(5):1896-901
Proceedings of the National Academy of Sciences of the United States of America 1997 Mar 4;94(5):1896-901
Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase.
Nauseef WM, McCormick SJ, Clark RA
The Journal of biological chemistry 1995 Mar 3;270(9):4741-7
The Journal of biological chemistry 1995 Mar 3;270(9):4741-7
Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase.
Nauseef WM, McCormick SJ, Clark RA
The Journal of biological chemistry 1995 Mar 3;270(9):4741-7
The Journal of biological chemistry 1995 Mar 3;270(9):4741-7
hsp108,,,,,, a novel heat shock inducible protein of chicken.
Sargan DR, Tsai MJ, O'Malley BW
Biochemistry 1986 Oct 7;25(20):6252-8
Biochemistry 1986 Oct 7;25(20):6252-8
Structural analysis of chicken oviduct progesterone receptor using monoclonal antibodies to the subunit B protein.
Edwards DP, Weigel NL, Schrader WT, O'Malley BW, McGuire WL
Biochemistry 1984 Sep 11;23(19):4427-35
Biochemistry 1984 Sep 11;23(19):4427-35
Structural analysis of chicken oviduct progesterone receptor using monoclonal antibodies to the subunit B protein.
Edwards DP, Weigel NL, Schrader WT, O'Malley BW, McGuire WL
Biochemistry 1984 Sep 11;23(19):4427-35
Biochemistry 1984 Sep 11;23(19):4427-35
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of Glucose-Regulated Protein 94 (Grp94) was performed by loading 2-fold serial dilutions of HeLa cell lysate, starting at 10 ug, per well onto a 4-20% Tris-HCl polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane and blocked with 5% non-fat dry milk in PBST for at least 1 hour. The membrane was probed with a Grp94 monoclonal antibody antibody (Product # MA3-016) at a dilution of 1:1000 overnight at 4ºC on a rocking platform, washed in TBS-0.1%Tween-20, and probed with an HRP-conjugated goat anti-rat IgG secondary antibody (Product # 31470) at a dilution of 1:25,000 for at least 1 hour. Chemiluminescent detection was performed using ECL Substrate (Product # 32132).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Western blot analysis of GRP94 was performed by loading 25 µg of Hela (lane 1), C6 (lane 2) and NIH-3T3 (lane 3) lysates onto an SDS polyacrylamide gel. Proteins were transferred to a PVDF membrane and blocked at 4ºC overnight. The membrane was probed with a GRP94 monoclonal antibody (Product # MA3-016) at a dilution of 1:200 overnight at 4°C, washed in TBST, and probed with an HRP-conjugated secondary antibody for 1 hr at room temperature in the dark. Chemiluminescent detection was performed using Pierce ECL Plus Western Blotting Substrate (Product # 32132). Results show a band at ~108 kDa using an exposure time of 4 min (Hela, C6) and 30 sec (NIH-3T3).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
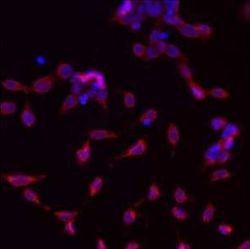
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
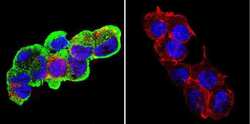
- Experimental details
- Immunofluorescent analysis of Glucose-Regulated Protein 94 using Glucose-Regulated Protein 94 Monoclonal Antibody (9G10) (Product # MA3-016) shows staining in C6 Cells. Glucose-Regulated Protein 94 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucose-Regulated Protein 94 (Product # MA3-016) at a dilution of 1:100 over night at 4ºC, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
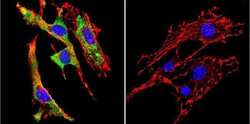
- Experimental details
- Immunofluorescent analysis of Glucose-Regulated Protein 94 using Glucose-Regulated Protein 94 Monoclonal Antibody (9G10) (Product # MA3-016) shows staining in Hela Cells. Glucose-Regulated Protein 94 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucose-Regulated Protein 94 (Product # MA3-016) at a dilution of 1:100 over night at 4ºC, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
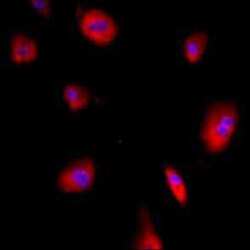
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
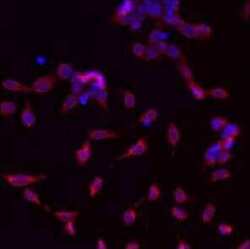
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
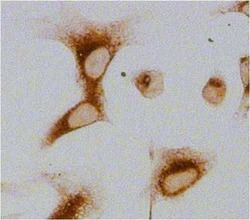
- Experimental details
- Immunocytochemistry analysis of Glucose-Regulated Protein 94 (Grp94) in U2OS cells. Cells fixed with 4% paraformaldehyde were permeabilized with 0.1% Triton X-100 (Product # 28314) in PBS for 15 minutes at room temperature and blocked with 2% BSA in PBST (from Product # 37525) for 30 minutes at room temperature. Cells were treated with Peroxidase Suppressor (Product # 35000), probed with a Grp94 monoclonal antibody (Product # MA3-016) at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBST, and incubated with an HRP-conjugated goat anti-rat IgG (H+L) secondary antibody (Product # 31471) at a dilution of 1:1000 for 30 minutes at room temperature. Chromogenic detection was performed using Metal Enhanced DAB Substrate Kit (Product # 34065). Images were taken on a Zeiss Axio Observer microscope at 20X magnification (x1.6 Optovar ~ 32X).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
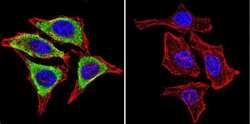
- Experimental details
- Immunofluorescent analysis of Glucose-Regulated Protein 94 using Glucose-Regulated Protein 94 Monoclonal Antibody (9G10) (Product # MA3-016) shows staining in NIH-3T3 Cells. Glucose-Regulated Protein 94 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucose-Regulated Protein 94 (Product # MA3-016) at a dilution of 1:20 over night at 4ºC, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
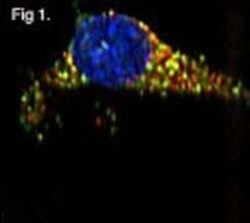
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
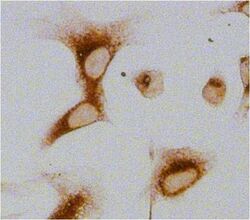
- Experimental details
- Immunocytochemistry analysis of Glucose-Regulated Protein 94 (Grp94) in U2OS cells. Cells fixed with 4% paraformaldehyde were permeabilized with 0.1% Triton X-100 (Product # 28314) in PBS for 15 minutes at room temperature and blocked with 2% BSA in PBST (from Product # 37525) for 30 minutes at room temperature. Cells were treated with Peroxidase Suppressor (Product # 35000), probed with a Grp94 monoclonal antibody (Product # MA3-016) at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBST, and incubated with an HRP-conjugated goat anti-rat IgG (H+L) secondary antibody (Product # 31471) at a dilution of 1:1000 for 30 minutes at room temperature. Chromogenic detection was performed using Metal Enhanced DAB Substrate Kit (Product # 34065). Images were taken on a Zeiss Axio Observer microscope at 20X magnification (x1.6 Optovar ~ 32X).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
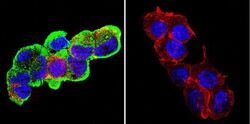
- Experimental details
- Immunofluorescent analysis of Glucose-Regulated Protein 94 using Glucose-Regulated Protein 94 Monoclonal Antibody (9G10) (Product # MA3-016) shows staining in C6 Cells. Glucose-Regulated Protein 94 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucose-Regulated Protein 94 (Product # MA3-016) at a dilution of 1:100 over night at 4ºC, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
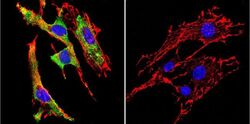
- Experimental details
- Immunofluorescent analysis of Glucose-Regulated Protein 94 using Glucose-Regulated Protein 94 Monoclonal Antibody (9G10) (Product # MA3-016) shows staining in Hela Cells. Glucose-Regulated Protein 94 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucose-Regulated Protein 94 (Product # MA3-016) at a dilution of 1:100 over night at 4ºC, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
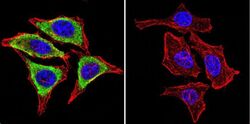
- Experimental details
- Immunofluorescent analysis of Glucose-Regulated Protein 94 using Glucose-Regulated Protein 94 Monoclonal Antibody (9G10) (Product # MA3-016) shows staining in NIH-3T3 Cells. Glucose-Regulated Protein 94 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucose-Regulated Protein 94 (Product # MA3-016) at a dilution of 1:20 over night at 4ºC, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
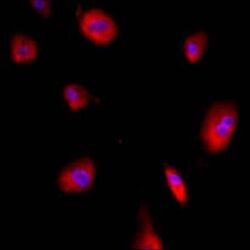
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
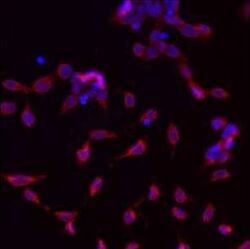
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of GRP94 using a monoclonal antibody (Product # MA3-016).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
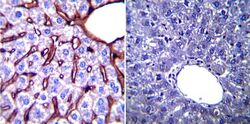
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse liver tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Glucose Regulated Protein 94 (Product # MA3-016) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
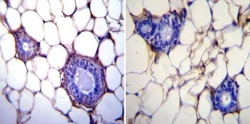
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse breast tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Glucose Regulated Protein 94 (Product # MA3-016) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
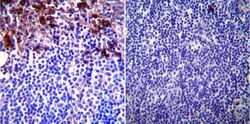
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse lymph node. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Glucose Regulated Protein 94 (Product # MA3-016) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
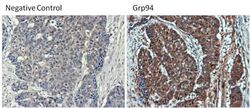
- Experimental details
- Immunohistochemistry was performed on poorly differentiated adenocarcinoma from paraffin-embedded human colon tissue sections. To expose target proteins, heat-induced epitope retrieval was performed using 10mM sodium citrate (pH 6.0) buffer for 20 minutes at 95ºC. Following antigen retrieval, tissues were blocked in 3% BSA (Product # 37525) in PBST for 30 minutes at room temperature and then probed with a Grp94 monoclonal antibody (Product # MA3-016) at a dilution of 1:100 for 1 hour in a humidified chamber (right panel). As a negative control, the primary antibody was eliminated from the staining procedure (left panel). Tissues were washed extensively with PBS/0.025% Tween-20 (Product # 28314) and endogenous peroxidase activity quenched with Peroxidase Suppressor (Product # 35000) for 30 minutes at room temperature. Detection was performed using an HRP-conjugated goat anti-rat IgG-HRP secondary antibody (Product # 31470) at a dilution of 1:500 followed by colorimetric detection using Metal Enhanced DAB Substrate Kit (Product # 34065). Tissues were counterstained with hematoxylin and prepped for mouting. Images were taken on a Zeiss Axiovision microscope at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
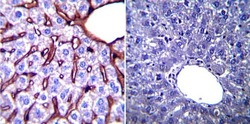
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse liver tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Glucose Regulated Protein 94 (Product # MA3-016) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
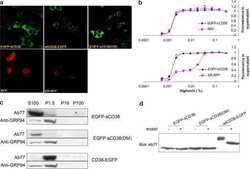
- Figure 1. RegDCs EXO protect encapsulated immunoregulatory cargo (TGFB1 and IL10) from proteolytic degradation . (A) Nano-tracking analysis to determine EXO number and size distribution in nm. (B) Transmission electron microscopy (TEM) to visualize EXO shape and immuno-gold TEM to detect EXO marker tetraspanin CD63, showing unstained (left) and positive staining (right)(arrows). (C) Western blotting to detect other EXO-related markers including CD81, TSG101, GRP94 and B-actin in donor DCs and EXO (left) and anti/pro-inflammatory cytokines including TGFB1, IL10, IL6, IL1B and TNF and the costimulatory molecule CD86 as well as EXO associated proteins ALIX and TSG101 in DCs EXO subsets (right). (D) TGFB1 and IL10 content of lysed EXO and in regDCs EXO supernatant by ELISA. (E) TGFB1 and IL10 content of non-lysed EXO (transmembrane domain) by ELISA. (F) Immunogold TEM to detect luminal and transmembrane TGFB1 in regDCs EXO. (G) regDCs EXO (left) or equivalent concentration of free TGFB1 and IL10 (right) were treated with trypsin (upper panel) or proteinase-K (lower panel) and incubated in control buffer (1hour at 37degC) and analysed by western blotting to detect the levels of TGFB1, IL10 and exosomal markers TSG101 (upper panel) and CD81 (lower panel).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
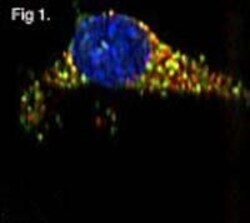
- Fig. 5 Increased intracellular damages after AUY-VE. A673 cells were treated with 45 nM AUY922 +- 2 uM VE821 for 24 h. a Intracellular structures were analyzed by transmission electron microscopy (TEM). Red arrows indicate lipid-filled vesicles; yellow arrows indicate lysosomes. b Golgi networks were analyzed by fluorescence microscopy of GM130. Red arrows indicate dispersed Golgi structures. c Endoplasmic reticulum (ER) networks were analyzed by fluorescence microscopy of GRP94. Red arrows indicate vesicular ER structures. A673 cells were treated with 1-2 ug/ml of tunicamycin for 24 h. e ER networks were analyzed by fluorescence microscopy of GRP94. c - e DAPI was used to stain cell nuclei. A shows one representative experiments; B-E are representative for at least two independent experiments
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation