Antibody data
- Antibody Data
- Antigen structure
- References [42]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [1]
- Immunohistochemistry [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- HPA008784 - Provider product page

- Provider
- Atlas Antibodies
- Proper citation
- Atlas Antibodies Cat#HPA008784, RRID:AB_1849181
- Product name
- Anti-FUS
- Antibody type
- Polyclonal
- Description
- Polyclonal Antibody against Human FUS, Gene description: FUS RNA binding protein, Alternative Gene Names: ALS6, FUS1, hnRNP-P2, HNRNPP2, TLS, Validated applications: ICC, IHC, WB, Uniprot ID: P35637, Storage: Store at +4°C for short term storage. Long time storage is recommended at -20°C.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Conjugate
- Unconjugated
- Isotype
- IgG
- Vial size
- 100 µl
- Concentration
- 0.2 mg/ml
- Storage
- Store at +4°C for short term storage. Long time storage is recommended at -20°C.
- Handling
- The antibody solution should be gently mixed before use.
Submitted references Psychiatric symptoms of frontotemporal dementia and subcortical (co-)pathology burden: new insights
Targeting RACK1 to alleviate TDP-43 and FUS proteinopathy-mediated suppression of protein translation and neurodegeneration
Neuronal dysfunction caused by FUSR521G promotes ALS-associated phenotypes that are attenuated by NF-κB inhibition
Acid sphingomyelinase inhibition improves motor behavioral deficits and neuronal loss in an amyotrophic lateral sclerosis mouse model
NUP62 localizes to ALS/FTLD pathological assemblies and contributes to TDP-43 insolubility
Proteomic analysis of heat-stable proteins revealed an increased proportion of proteins with compositionally biased regions
A postzygotic de novo NCDN mutation identified in a sporadic FTLD patient results in neurochondrin haploinsufficiency and altered FUS granule dynamics
A novel temporal‐predominant neuro‐astroglial tauopathy associated with TMEM106B gene polymorphism in FTLD/ALS‐TDP
Interactions between ALS-linked FUS and nucleoporins are associated with defects in the nucleocytoplasmic transport pathway
Quantifying the phase separation property of chromatin-associated proteins under physiological conditions using an anti-1,6-hexanediol index
Young-onset frontotemporal dementia with FUS pathology
Disruption of orbitofrontal-hypothalamic projections in a murine ALS model and in human patients
Frontotemporal Dementia: Correlations Between Psychiatric Symptoms and Pathology
Quantitative patterns of motor cortex proteinopathy across ALS genotypes
Factors associated with development and distribution of granular/fuzzy astrocytes in neurodegenerative diseases
Spectrum of tau pathologies in Huntington's disease
FUS-mediated regulation of acetylcholine receptor transcription at neuromuscular junctions is compromised in amyotrophic lateral sclerosis
Synaptic FUS Localization During Motoneuron Development and Its Accumulation in Human ALS Synapses
An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function
Super-Resolution Microscopy Reveals Presynaptic Localization of the ALS/FTD Related Protein FUS in Hippocampal Neurons
Chorea as a clinical feature of the basophilic inclusion body disease subtype of fused-in-sarcoma-associated frontotemporal lobar degeneration
Familial Behavioral Variant Frontotemporal Dementia Associated With Astrocyte-Predominant Tauopathy
Neuropathologic analysis of Tyr69His TTR variant meningovascular amyloidosis with dementia
A truncating SOD1 mutation, p.Gly141X, is associated with clinical and pathologic heterogeneity, including frontotemporal lobar degeneration
Amelioration of toxicity in neuronal models of amyotrophic lateral sclerosis by hUPF1
Optineurin immunoreactivity in neuronal and glial intranuclear inclusions in adult-onset neuronal intranuclear inclusion disease.
Immunoreactivity of valosin-containing protein in sporadic amyotrophic lateral sclerosis and in a case of its novel mutant
ALS-associated mutation FUS-R521C causes DNA damage and RNA splicing defects
Activity-dependent FUS dysregulation disrupts synaptic homeostasis
Frontotemporal dementia–amyotrophic lateral sclerosis syndrome locus on chromosome 16p12.1–q12.2: genetic, clinical and neuropathological analysis
Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS
Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations
Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations
FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations
A comparative clinical, pathological, biochemical and genetic study of fused in sarcoma proteinopathies
Distribution and Pattern of Pathology in Subjects with Familial or Sporadic Late-Onset Cerebellar Ataxia as Assessed by p62/Sequestosome Immunohistochemistry
FUS‐immunoreactive inclusions are a common feature in sporadic and non‐SOD1 familial amyotrophic lateral sclerosis
FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration
Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration
A new subtype of frontotemporal lobar degeneration with FUS pathology
FUS pathology in basophilic inclusion body disease
Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease
Scarioni M, Gami-Patel P, Peeters C, de Koning F, Seelaar H, Mol M, van Swieten J, Rozemuller A, Hoozemans J, Pijnenburg Y, Dijkstra A
Brain 2023;146(1):307-320
Brain 2023;146(1):307-320
Targeting RACK1 to alleviate TDP-43 and FUS proteinopathy-mediated suppression of protein translation and neurodegeneration
Zhao B, Cowan C, Coutts J, Christy D, Saraph A, Hsueh S, Plotkin S, Mackenzie I, Kaplan J, Cashman N
Acta Neuropathologica Communications 2023;11(1)
Acta Neuropathologica Communications 2023;11(1)
Neuronal dysfunction caused by FUSR521G promotes ALS-associated phenotypes that are attenuated by NF-κB inhibition
Pelaez M, Desmeules A, Gelon P, Glasson B, Marcadet L, Rodgers A, Phaneuf D, Pozzi S, Dutchak P, Julien J, Sephton C
Acta Neuropathologica Communications 2023;11(1)
Acta Neuropathologica Communications 2023;11(1)
Acid sphingomyelinase inhibition improves motor behavioral deficits and neuronal loss in an amyotrophic lateral sclerosis mouse model
Choi B, Park K, Park M, Huang E, Kim S, Bae J, Jin H
BMB Reports 2022;55(12):621-626
BMB Reports 2022;55(12):621-626
NUP62 localizes to ALS/FTLD pathological assemblies and contributes to TDP-43 insolubility
Gleixner A, Verdone B, Otte C, Anderson E, Ramesh N, Shapiro O, Gale J, Mauna J, Mann J, Copley K, Daley E, Ortega J, Cicardi M, Kiskinis E, Kofler J, Pandey U, Trotti D, Donnelly C
Nature Communications 2022;13(1)
Nature Communications 2022;13(1)
Proteomic analysis of heat-stable proteins revealed an increased proportion of proteins with compositionally biased regions
Park H, Yamanaka T, Nukina N
Scientific Reports 2022;12(1)
Scientific Reports 2022;12(1)
A postzygotic de novo NCDN mutation identified in a sporadic FTLD patient results in neurochondrin haploinsufficiency and altered FUS granule dynamics
Nicolas G, Sévigny M, Lecoquierre F, Marguet F, Deschênes A, del Pelaez M, Feuillette S, Audebrand A, Lecourtois M, Rousseau S, Richard A, Cassinari K, Deramecourt V, Duyckaerts C, Boland A, Deleuze J, Meyer V, Clarimon Echavarria J, Gelpi E, Akiyama H, Hasegawa M, Kawakami I, Wong T, Van Rooij J, Van Swieten J, Campion D, Dutchak P, Wallon D, Lavoie-Cardinal F, Laquerrière A, Rovelet-Lecrux A, Sephton C
Acta Neuropathologica Communications 2022;10(1)
Acta Neuropathologica Communications 2022;10(1)
A novel temporal‐predominant neuro‐astroglial tauopathy associated with TMEM106B gene polymorphism in FTLD/ALS‐TDP
Llibre‐Guerra J, Lee S, Suemoto C, Ehrenberg A, Kovacs G, Karydas A, Staffaroni A, Franca Resende E, Kim E, Hwang J, Ramos E, Wojta K, Pasquini L, Pang S, Spina S, Allen I, Kramer J, Miller B, Seeley W, Grinberg L
Brain Pathology 2021;31(2):267-282
Brain Pathology 2021;31(2):267-282
Interactions between ALS-linked FUS and nucleoporins are associated with defects in the nucleocytoplasmic transport pathway
Lin Y, Kumar M, Ramesh N, Anderson E, Nguyen A, Kim B, Cheung S, McDonough J, Skarnes W, Lopez-Gonzalez R, Landers J, Fawzi N, Mackenzie I, Lee E, Nickerson J, Grunwald D, Pandey U, Bosco D
Nature Neuroscience 2021;24(8):1077-1088
Nature Neuroscience 2021;24(8):1077-1088
Quantifying the phase separation property of chromatin-associated proteins under physiological conditions using an anti-1,6-hexanediol index
Shi M, You K, Chen T, Hou C, Liang Z, Liu M, Wang J, Wei T, Qin J, Chen Y, Zhang M, Li T
Genome Biology 2021;22(1)
Genome Biology 2021;22(1)
Young-onset frontotemporal dementia with FUS pathology
Gowell M, Baker I, Ansorge O, Husain M
Practical Neurology 2021;21(2):149-152
Practical Neurology 2021;21(2):149-152
Disruption of orbitofrontal-hypothalamic projections in a murine ALS model and in human patients
Bayer D, Antonucci S, Müller H, Saad R, Dupuis L, Rasche V, Böckers T, Ludolph A, Kassubek J, Roselli F
Translational Neurodegeneration 2021;10(1)
Translational Neurodegeneration 2021;10(1)
Frontotemporal Dementia: Correlations Between Psychiatric Symptoms and Pathology
Scarioni M, Gami‐Patel P, Timar Y, Seelaar H, van Swieten J, Rozemuller A, Dols A, Scarpini E, Galimberti D, Hoozemans J, Pijnenburg Y, Dijkstra A
Annals of Neurology 2020;87(6):950-961
Annals of Neurology 2020;87(6):950-961
Quantitative patterns of motor cortex proteinopathy across ALS genotypes
Nolan M, Scott C, Gamarallage M, Lunn D, Carpenter K, McDonough E, Meyer D, Kaanumalle S, Santamaria-Pang A, Turner M, Talbot K, Ansorge O
Acta Neuropathologica Communications 2020;8(1)
Acta Neuropathologica Communications 2020;8(1)
Factors associated with development and distribution of granular/fuzzy astrocytes in neurodegenerative diseases
Miki T, Yokota O, Haraguchi T, Ishizu H, Hasegawa M, Ishihara T, Ueno S, Takenoshita S, Terada S, Yamada N
Brain Pathology 2020;30(4):811-830
Brain Pathology 2020;30(4):811-830
Spectrum of tau pathologies in Huntington's disease
Baskota S, Lopez O, Greenamyre J, Kofler J
Laboratory Investigation 2019;99(7):1068-1077
Laboratory Investigation 2019;99(7):1068-1077
FUS-mediated regulation of acetylcholine receptor transcription at neuromuscular junctions is compromised in amyotrophic lateral sclerosis
Picchiarelli G, Demestre M, Zuko A, Been M, Higelin J, Dieterlé S, Goy M, Mallik M, Sellier C, Scekic-Zahirovic J, Zhang L, Rosenbohm A, Sijlmans C, Aly A, Mersmann S, Sanjuan-Ruiz I, Hübers A, Messaddeq N, Wagner M, van Bakel N, Boutillier A, Ludolph A, Lagier-Tourenne C, Boeckers T, Dupuis L, Storkebaum E
Nature Neuroscience 2019;22(11):1793-1805
Nature Neuroscience 2019;22(11):1793-1805
Synaptic FUS Localization During Motoneuron Development and Its Accumulation in Human ALS Synapses
Deshpande D, Higelin J, Schoen M, Vomhof T, Boeckers T, Demestre M, Michaelis J
Frontiers in Cellular Neuroscience 2019;13
Frontiers in Cellular Neuroscience 2019;13
An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function
Mateju D, Franzmann T, Patel A, Kopach A, Boczek E, Maharana S, Lee H, Carra S, Hyman A, Alberti S
The EMBO Journal 2017;36(12):1669-1687
The EMBO Journal 2017;36(12):1669-1687
Super-Resolution Microscopy Reveals Presynaptic Localization of the ALS/FTD Related Protein FUS in Hippocampal Neurons
Schoen M, Reichel J, Demestre M, Putz S, Deshpande D, Proepper C, Liebau S, Schmeisser M, Ludolph A, Michaelis J, Boeckers T
Frontiers in Cellular Neuroscience 2016;9
Frontiers in Cellular Neuroscience 2016;9
Chorea as a clinical feature of the basophilic inclusion body disease subtype of fused-in-sarcoma-associated frontotemporal lobar degeneration
Kawakami I, Kobayashi Z, Arai T, Yokota O, Nonaka T, Aoki N, Niizato K, Oshima K, Higashi S, Katsuse O, Hosokawa M, Hasegawa M, Akiyama H
Acta Neuropathologica Communications 2016;4(1)
Acta Neuropathologica Communications 2016;4(1)
Familial Behavioral Variant Frontotemporal Dementia Associated With Astrocyte-Predominant Tauopathy
Ferrer I, Legati A, García-Monco J, Gomez-Beldarrain M, Carmona M, Blanco R, Seeley W, Coppola G
Journal of Neuropathology & Experimental Neurology 2015;74(4):370-379
Journal of Neuropathology & Experimental Neurology 2015;74(4):370-379
Neuropathologic analysis of Tyr69His TTR variant meningovascular amyloidosis with dementia
Ziskin J, Greicius M, Zhu W, Okumu A, Adams C, Plowey E
Acta Neuropathologica Communications 2015;3(1)
Acta Neuropathologica Communications 2015;3(1)
A truncating SOD1 mutation, p.Gly141X, is associated with clinical and pathologic heterogeneity, including frontotemporal lobar degeneration
Nakamura M, Bieniek K, Lin W, Graff-Radford N, Murray M, Castanedes-Casey M, Desaro P, Baker M, Rutherford N, Robertson J, Rademakers R, Dickson D, Boylan K
Acta Neuropathologica 2015;130(1):145-157
Acta Neuropathologica 2015;130(1):145-157
Amelioration of toxicity in neuronal models of amyotrophic lateral sclerosis by hUPF1
Barmada S, Ju S, Arjun A, Batarse A, Archbold H, Peisach D, Li X, Zhang Y, Tank E, Qiu H, Huang E, Ringe D, Petsko G, Finkbeiner S
Proceedings of the National Academy of Sciences 2015;112(25):7821-7826
Proceedings of the National Academy of Sciences 2015;112(25):7821-7826
Optineurin immunoreactivity in neuronal and glial intranuclear inclusions in adult-onset neuronal intranuclear inclusion disease.
Nakamura M, Murray ME, Lin WL, Kusaka H, Dickson DW
American journal of neurodegenerative disease 2014;3(2):93-102
American journal of neurodegenerative disease 2014;3(2):93-102
Immunoreactivity of valosin-containing protein in sporadic amyotrophic lateral sclerosis and in a case of its novel mutant
Ayaki T, Ito H, Fukushima H, Inoue T, Kondo T, Ikemoto A, Asano T, Shodai A, Fujita T, Fukui S, Morino H, Nakano S, Kusaka H, Yamashita H, Ihara M, Matsumoto R, Kawamata J, Urushitani M, Kawakami H, Takahashi R
Acta Neuropathologica Communications 2014;2(1)
Acta Neuropathologica Communications 2014;2(1)
ALS-associated mutation FUS-R521C causes DNA damage and RNA splicing defects
Qiu H, Lee S, Shang Y, Wang W, Au K, Kamiya S, Barmada S, Finkbeiner S, Lui H, Carlton C, Tang A, Oldham M, Wang H, Shorter J, Filiano A, Roberson E, Tourtellotte W, Chen B, Tsai L, Huang E
Journal of Clinical Investigation 2014;124(3):981-999
Journal of Clinical Investigation 2014;124(3):981-999
Activity-dependent FUS dysregulation disrupts synaptic homeostasis
Sephton C, Tang A, Kulkarni A, West J, Brooks M, Stubblefield J, Liu Y, Zhang M, Green C, Huber K, Huang E, Herz J, Yu G
Proceedings of the National Academy of Sciences 2014;111(44)
Proceedings of the National Academy of Sciences 2014;111(44)
Frontotemporal dementia–amyotrophic lateral sclerosis syndrome locus on chromosome 16p12.1–q12.2: genetic, clinical and neuropathological analysis
Dobson-Stone C, Luty A, Thompson E, Blumbergs P, Brooks W, Short C, Field C, Panegyres P, Hecker J, Solski J, Blair I, Fullerton J, Halliday G, Schofield P, Kwok J
Acta Neuropathologica 2013;125(4):523-533
Acta Neuropathologica 2013;125(4):523-533
Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS
Dormann D, Madl T, Valori C, Bentmann E, Tahirovic S, Abou-Ajram C, Kremmer E, Ansorge O, Mackenzie I, Neumann M, Haass C
The EMBO Journal 2012;31(22):4258-4275
The EMBO Journal 2012;31(22):4258-4275
Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations
Snowden J, Rollinson S, Thompson J, Harris J, Stopford C, Richardson A, Jones M, Gerhard A, Davidson Y, Robinson A, Gibbons L, Hu Q, DuPlessis D, Neary D, Mann D, Pickering-Brown S
Brain 2012;135(3):693-708
Brain 2012;135(3):693-708
Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations
Kino Y, Washizu C, Aquilanti E, Okuno M, Kurosawa M, Yamada M, Doi H, Nukina N
Nucleic Acids Research 2011;39(7):2781-2798
Nucleic Acids Research 2011;39(7):2781-2798
FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations
Neumann M, Bentmann E, Dormann D, Jawaid A, DeJesus-Hernandez M, Ansorge O, Roeber S, Kretzschmar H, Munoz D, Kusaka H, Yokota O, Ang L, Bilbao J, Rademakers R, Haass C, Mackenzie I
Brain 2011;134(9):2595-2609
Brain 2011;134(9):2595-2609
A comparative clinical, pathological, biochemical and genetic study of fused in sarcoma proteinopathies
Lashley T, Rohrer J, Bandopadhyay R, Fry C, Ahmed Z, Isaacs A, Brelstaff J, Borroni B, Warren J, Troakes C, King A, Al-Saraj S, Newcombe J, Quinn N, Ostergaard K, Schroder H, Bojsen-Moller M, Braendgaard H, Fox N, Rossor M, Lees A, Holton J, Revesz T
Brain 2011;134(9):2548-2564
Brain 2011;134(9):2548-2564
Distribution and Pattern of Pathology in Subjects with Familial or Sporadic Late-Onset Cerebellar Ataxia as Assessed by p62/Sequestosome Immunohistochemistry
Pikkarainen M, Hartikainen P, Soininen H, Alafuzoff I
The Cerebellum 2011;10(4):720-731
The Cerebellum 2011;10(4):720-731
FUS‐immunoreactive inclusions are a common feature in sporadic and non‐SOD1 familial amyotrophic lateral sclerosis
Deng H, Zhai H, Bigio E, Yan J, Fecto F, Ajroud K, Mishra M, Ajroud‐Driss S, Heller S, Sufit R, Siddique N, Mugnaini E, Siddique T
Annals of Neurology 2010;67(6):739-748
Annals of Neurology 2010;67(6):739-748
FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration
Urwin H, Josephs K, Rohrer J, Mackenzie I, Neumann M, Authier A, Seelaar H, Van Swieten J, Brown J, Johannsen P, Nielsen J, Holm I, Dickson D, Rademakers R, Graff-Radford N, Parisi J, Petersen R, Hatanpaa K, White III C, Weiner M, Geser F, Van Deerlin V, Trojanowski J, Miller B, Seeley W, van der Zee J, Kumar-Singh S, Engelborghs S, De Deyn P, Van Broeckhoven C, Bigio E, Deng H, Halliday G, Kril J, Munoz D, Mann D, Pickering-Brown S, Doodeman V, Adamson G, Ghazi-Noori S, Fisher E, Holton J, Revesz T, Rossor M, Collinge J, Mead S, Isaacs A
Acta Neuropathologica 2010;120(1):33-41
Acta Neuropathologica 2010;120(1):33-41
Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration
Seelaar H, Klijnsma K, de Koning I, van der Lugt A, Chiu W, Azmani A, Rozemuller A, van Swieten J
Journal of Neurology 2009;257(5):747-753
Journal of Neurology 2009;257(5):747-753
A new subtype of frontotemporal lobar degeneration with FUS pathology
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar H, Mackenzie I
Brain 2009;132(11):2922-2931
Brain 2009;132(11):2922-2931
FUS pathology in basophilic inclusion body disease
Munoz D, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, Kuroda S, Mackenzie I
Acta Neuropathologica 2009;118(5):617-627
Acta Neuropathologica 2009;118(5):617-627
Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease
Neumann M, Roeber S, Kretzschmar H, Rademakers R, Baker M, Mackenzie I
Acta Neuropathologica 2009;118(5):605-616
Acta Neuropathologica 2009;118(5):605-616
No comments: Submit comment
Enhanced validation
- Submitted by
- Atlas Antibodies (provider)
- Enhanced method
- Genetic validation
- Main image

- Experimental details
- Western blot analysis in U-251MG cells transfected with control siRNA, target specific siRNA probe #1 and #2, using Anti-FUS antibody. Remaining relative intensity is presented. Loading control: Anti-GAPDH.
- Sample type
- Human
- Protocol
- Protocol
Supportive validation
- Submitted by
- Atlas Antibodies (provider)
- Main image
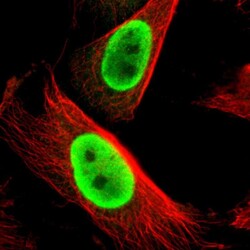
- Experimental details
- Immunofluorescent staining of human cell line U-251 MG shows localization to nucleoplasm.
- Sample type
- Human
Supportive validation
- Submitted by
- Atlas Antibodies (provider)
- Enhanced method
- Orthogonal validation
- Main image
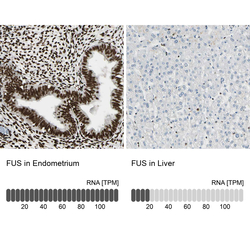
- Experimental details
- Immunohistochemistry analysis in human endometrium and liver tissues using HPA008784 antibody. Corresponding FUS RNA-seq data are presented for the same tissues.
- Sample type
- Human
- Protocol
- Protocol
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry