Antibody data
- Antibody Data
- Antigen structure
- References [0]
- Comments [0]
- Validations
- Flow cytometry [3]
Submit
Validation data
Reference
Comment
Report error
- Product number
- H001T02Y02-A - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD4 Monoclonal Antibody (SK3 (SK-3)), NovaFluor™ Yellow 590, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The SK3 monoclonal antibody reacts with human CD4, a 59-kDa cell surface receptor expressed by a majority of thymocytes, a subpopulation of mature T helper cells, and at low levels on monocytes. CD4 is a receptor for the human immunodeficiency virus (HIV). SK3 blocks HIV binding and mixed lymphocyte reaction. The SK3 and RPA-T4 monoclonal antibodies do not cross-block binding, suggesting recognition of distinct epitopes. Each product contains 1 vial of NovaFluor conjugate and 1 vial of CellBlox Plus Blocking Buffer . Applications Reported: This SK3 (SK-3) antibody has been reported for use in flow cytometric analysis. Applications Tested: This SK3 (SK-3) antibody has been pre-titrated and tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 µL (0.1 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. NovaFluor dyes are not compatible with DNA intercalating viability dyes. Do not use viability dyes such as propidium iodide, 7-actinomycin D (7-AAD) and DAPI. Invitrogen LIVE/DEAD Fixable Dead Cell stains are recommended for use with NovaFluor dyes. This NovaFluor conjugate has been updated to ship with CellBlox Plus Blocking Buffer (Cat. No. (C001T06F01)). This buffer contains formulation improvements over CellBlox. CellBlox Plus Blocking Buffer is required for optimal staining with NovaFluor conjugates and should be used in all experiments where NovaFluor conjugates are used. Whenever possible, we recommend adding CellBlox Plus Blocking Buffer to antibody cocktails/master mixes prior to combining with cells. Add 5 µL per sample (regardless of the number of NovaFluors in your panel) to use the antibody cocktail as intended. For single-color controls, use 5 µL of CellBlox Blocking Buffer per 100 µL of cell sample containing 10^3 to 10^8 cells. NovaFluor conjugates are based on Phiton technology utilizing novel nucleic acid dye structures that allow for engineered fluorescent signatures with consideration for spillover and spread impacts. Learn more Excitation: 552 nm; Emission: 592 nm; Laser: 561 nm (Yellow) Laser
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- SK3 (SK-3)
- Vial size
- 25 Tests
- Concentration
- 2 µL/Test
- Storage
- 4°C, store in dark, DO NOT FREEZE!
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Normal human peripheral blood cells were unstained (left) or stained with CD4 Monoclonal Antibody, NovaFluor Yellow 590 (right). All cells were co-stained with CD8a Monoclonal Antibody, eFluor 450 (Product # 48-0086-42). Total viable cells in the lymphocyte gate were used for analysis, as determined by LIVE/DEAD Blue (Product # L34962). Data was acquired on a 5-laser Cytek Aurora and unmixed with autofluorescence extraction.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
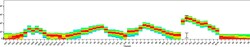
- Experimental details
- Spectral signature for NovaFluor Yellow 590 collected on a 5-laser Cytek Aurora Full Spectrum flow cytometer using Cytek assay settings. Human peripheral blood mononuclear cells were stained with anti-human CD4 (SK3) and signatures displayed following gating on the lymphocyte population.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
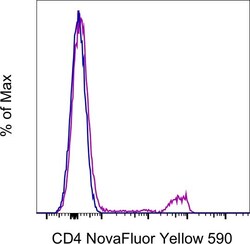
- Experimental details
- Normal human PBMCs were either left unstained (blue histogram) or stained with CD4 Monoclonal Antibody, NovaFluor Yellow 590 (purple histogram) and acquired in the YG2 channel on a 5-laser Cytek Aurora. Cells in the lymphocyte gate were used in the analysis.
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry