Antibody data
- Antibody Data
- Antigen structure
- References [8]
- Comments [0]
- Validations
- Immunoprecipitation [1]
- Immunohistochemistry [3]
- Other assay [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-2024 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- NONO Monoclonal Antibody (78-1 C)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- MA3-2024 detects the nmt55/p54nrb in human, rat and rabbit samples. MA3-2024 has been successfully used in immunohistochemistry, immunoprecipitation, and Western blot procedures. By Western blot, this antibody detects a ~55 kDa protein representing nmt55/p54nrb in MCF-7 cells, human breast tumor cells, rat uterine tissue, and rabbit liver extracts. In immunohistochemistry this antibody recognizes the nmt55/p54nrb in MCF-7 cells and human breast tumor cells. This monoclonal antibody has been referred to as NMT-1. The MA3-2024 immunogen is a synthetic peptide corresponding to residues T(140) V R E A G P P A F Y R P N S(154) of human ER alpha.
- Reactivity
- Human, Rat, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 78-1 C
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references TRAP150 interacts with the RNA-binding domain of PSF and antagonizes splicing of numerous PSF-target genes in T cells.
Role of NonO-histone interaction in TNFalpha-suppressed prolyl-4-hydroxylase alpha1.
Combinatorial control of signal-induced exon repression by hnRNP L and PSF.
p54nrb is a component of the snRNP-free U1A (SF-A) complex that promotes pre-mRNA cleavage during polyadenylation.
Multisite phosphorylation of Pin1-associated mitotic phosphoproteins revealed by monoclonal antibodies MPM-2 and CC-3.
Immunodetection of nmt55/p54nrb isoforms in human breast cancer.
Immunodetection of nmt55/p54nrb isoforms in human breast cancer.
The intracisternal A-particle proximal enhancer-binding protein activates transcription and is identical to the RNA- and DNA-binding protein p54nrb/NonO.
Yarosh CA, Tapescu I, Thompson MG, Qiu J, Mallory MJ, Fu XD, Lynch KW
Nucleic acids research 2015 Oct 15;43(18):9006-16
Nucleic acids research 2015 Oct 15;43(18):9006-16
Role of NonO-histone interaction in TNFalpha-suppressed prolyl-4-hydroxylase alpha1.
Zhang C, Zhang MX, Shen YH, Burks JK, Li XN, LeMaire SA, Yoshimura K, Aoki H, Matsuzaki M, An FS, Engler DA, Matsunami RK, Coselli JS, Zhang Y, Wang XL
Biochimica et biophysica acta 2008 Aug;1783(8):1517-28
Biochimica et biophysica acta 2008 Aug;1783(8):1517-28
Combinatorial control of signal-induced exon repression by hnRNP L and PSF.
Melton AA, Jackson J, Wang J, Lynch KW
Molecular and cellular biology 2007 Oct;27(19):6972-84
Molecular and cellular biology 2007 Oct;27(19):6972-84
p54nrb is a component of the snRNP-free U1A (SF-A) complex that promotes pre-mRNA cleavage during polyadenylation.
Liang S, Lutz CS
RNA (New York, N.Y.) 2006 Jan;12(1):111-21
RNA (New York, N.Y.) 2006 Jan;12(1):111-21
Multisite phosphorylation of Pin1-associated mitotic phosphoproteins revealed by monoclonal antibodies MPM-2 and CC-3.
Albert AL, Lavoie SB, Vincent M
BMC cell biology 2004 Jun 1;5:22
BMC cell biology 2004 Jun 1;5:22
Immunodetection of nmt55/p54nrb isoforms in human breast cancer.
Pavao M, Huang YH, Hafer LJ, Moreland RB, Traish AM
BMC cancer 2001;1:15
BMC cancer 2001;1:15
Immunodetection of nmt55/p54nrb isoforms in human breast cancer.
Pavao M, Huang YH, Hafer LJ, Moreland RB, Traish AM
BMC cancer 2001;1:15
BMC cancer 2001;1:15
The intracisternal A-particle proximal enhancer-binding protein activates transcription and is identical to the RNA- and DNA-binding protein p54nrb/NonO.
Basu A, Dong B, Krainer AR, Howe CC
Molecular and cellular biology 1997 Feb;17(2):677-86
Molecular and cellular biology 1997 Feb;17(2):677-86
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Immunoprecipitation of NONO was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of NONO monoclonal antibody (Product # MA3-2024) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a NONO monoclonal antibody (Product # MA3-2024) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
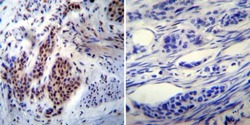
- Immunohistochemistry was performed on cancer biopsies of deparaffinized human Bladder carcinoma tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing nmt55/p54nrb (Product # MA3-2024) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
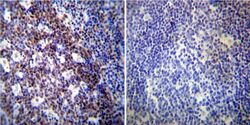
- Immunohistochemistry was performed on normal deparaffinized human Tonsil tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing nmt55/p54nrb (Product # MA3-2024) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
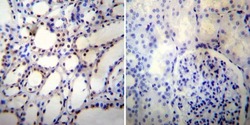
- Immunohistochemistry was performed on normal deparaffinized human Kidney tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:500 with a mouse monoclonal antibody recognizing nmt55/p54nrb (Product # MA3-2024) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
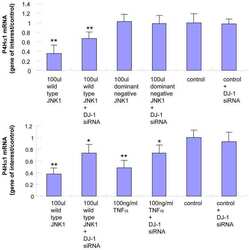
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
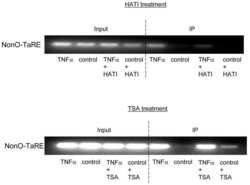
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
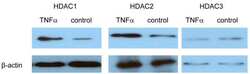
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
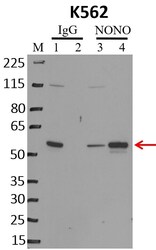
- Experimental details
- RNA immunoprecipitation (RIP) western of NONO was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of NONO monoclonal antibody (Product # MA3-2024) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a NONO monoclonal antibody (Product # MA3-2024) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
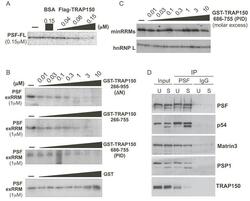
- Experimental details
- Figure 5. TRAP150 inhibits the RRM-dependent binding activity of PSF, and inhibition of binding requires more than the minimal RRM binding domain. ( A ) UV crosslinking of ESS1 RNA with full-length PSF (PSF-FL) either alone (-), in the presence of BSA as a control or in the presence of increasing amounts of full-length FLAG-tagged TRAP150. ( B ) Same as panel (A), but competing binding of exRRMs of PSF with indicated truncations of GST-TRAP150 or GST alone. ( C ) Same as panel (A) but competing for binding of the minRRMs and hnRNP L by GST-TRAP(PID). ( D ) Western blots of immunoprecipitation of PSF from unstimulated (TRAP150 bound) and stimulated (TRAP150 unbound) JSL1 cells showing relative binding of TRAP150 and other known PSF-interacting partners. The source of the doublet for PSPC1 and p54 nrb /NONO in stimulated cells is unknown, but is the same in input and IP samples.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation