PA5-28827
antibody from Invitrogen Antibodies
Targeting: CXCL13
ANGIE, ANGIE2, BCA-1, BLC, BLR1L, SCYB13
Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [4]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-28827 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CXCL13 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Recommended positive controls: CXCL13-transfected 293T. Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 1.21 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Poor clinical outcomes and immunoevasive contexture in CXCL13+CD8+ T cells enriched gastric cancer patients.
Jin K, Cao Y, Gu Y, Fang H, Fei Y, Wang J, Liu X, Lv K, He X, Lin C, Liu H, Li H, He H, Li R, Zhang H, Xu J
Oncoimmunology 2021 Apr 27;10(1):1915560
Oncoimmunology 2021 Apr 27;10(1):1915560
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
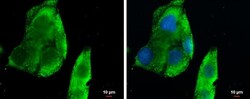
- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of CXCL13 was performed in HepG2 cells fixed in 4% paraformaldehyde at RT for 15 min. Green: CXCL13 Polyclonal Antibody (Product # PA5-28827) diluted at 1:500. Blue: Hoechst 33342 staining. Scale bar = 10 µm.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
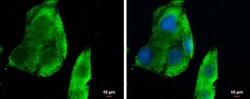
- Experimental details
- Immunocytochemistry-Immunofluorescence analysis of CXCL13 was performed in HepG2 cells fixed in 4% paraformaldehyde at RT for 15 min. Green: CXCL13 Polyclonal Antibody (Product # PA5-28827) diluted at 1:500. Blue: Hoechst 33342 staining. Scale bar = 10 µm.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
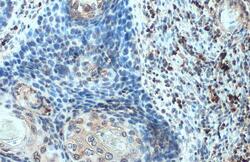
- Experimental details
- CXCL13 Polyclonal Antibody detects BCA1 protein at cell membrane and cytoplasm by immunohistochemical analysis. Sample: Paraffin-embedded human esophageal carcinoma. BCA1 stained by CXCL13 Polyclonal Antibody (Product # PA5-28827) diluted at 1:100. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
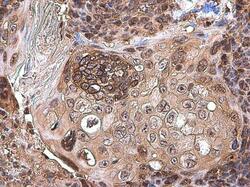
- Experimental details
- Immunohistochemistry (Paraffin) analysis of CXCL13 was performed in paraffin-embedded human esophageal carcinoma tissue using CXCL13 Polyclonal Antibody (Product # PA5-28827) at a dilution of 1:500.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
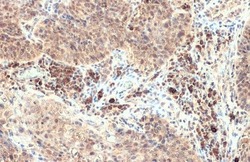
- Experimental details
- CXCL13 Polyclonal Antibody detects BCA1 protein at cytoplasm by immunohistochemical analysis. Sample: Paraffin-embedded human esophageal carcinoma. BCA1 stained by CXCL13 Polyclonal Antibody (Product # PA5-28827) diluted at 1:500. Antigen Retrieval: Citrate buffer, pH 6.0, 15 min.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
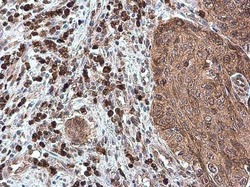
- Experimental details
- Immunohistochemistry (Paraffin) analysis of CXCL13 was performed in paraffin-embedded human esophageal carcinoma tissue using CXCL13 Polyclonal Antibody (Product # PA5-28827) at a dilution of 1:500.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
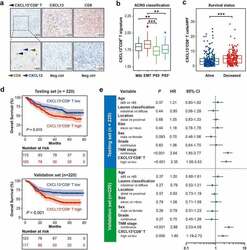
- Experimental details
- Figure 1. Intratumoral CXCL13 + CD8 + T cells predict poor prognosis in gastric cancer. (a) Immunohistochemical double-staining of CD8 (brown) and CXCL13 (blue) in gastric cancer tissues. Black arrowhead showed CXCL13 + CD8 + T cells (left panel). Immunohistochemistry (IHC) staining for CXCL13 (median panel), CD8 (right panel) and corresponding negative control were also shown. Neg ctrl refers to negative control. (b) Association between CXCL13 + CD8 + T cell signature and different ACRG classifications. Kruskal-Wallis test followed by Dunn's multiple comparisons test, * P < .05, ** P < .01, *** P < .001, ns refers to not significant. (c) Association between the number of CXCL13 + CD8 + T cells and patient survival outcomes. Mann-Whitney U test, * P < .05, ** P < .01, *** P < .001, ns refers to not significant. (d-e) Kaplan-Meier curves (d) for overall survival (OS) according to the number of intratumor CXCL13 + CD8 + T cells and multivariate analysis (e) based on clinicopathological characteristics in Testing set (n = 220) and Validation set (n = 220), respectively. The OS was compared between CXCL13 + CD8 + T high and CXCL13 + CD8 + T low subgroups. Log-rank test was performed for Kaplan-Meier curves. HR refers to hazard ratio, CI refers to confidence interval
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry