Antibody data
- Antibody Data
- Antigen structure
- References [0]
- Comments [0]
- Validations
- Immunocytochemistry [8]
- Immunoprecipitation [1]
- Immunohistochemistry [3]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-156 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Cyclin B2 Monoclonal Antibody (X29.2)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- MA1-156 detects Cyclin B2 in mammalian samples. MA1-156 has been successfully used in Immunofluorescence, Immunoprecipitation, IHC (P) and Western Blot procedures. Immunoprecipitation and Western Blot analysis with MA1-156 show the accumulation of a prominent band at ~51 kDa in camptothecin and hydroxyurea treated cells. MA1-156 also detects additional unknown band at ~80 kDa. In Immunofluorescence applications, MA1-156 shows cyclin B2 staining consistent with the Golgi region, whereas MA1-155 shows cyclin B1 co-localization with microtubules. MA1-156 reacts with Cyclin B2 from Xenopus laevis and mammalian sources. In Western blot applications, X29.2 also cross reacts with Cyclin B1.
- Reactivity
- Human, Mouse, Xenopus
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- X29.2
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
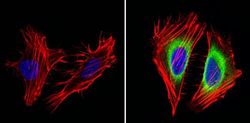
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) showing staining in the in the cytoplasm of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
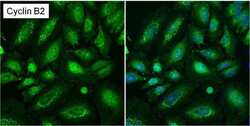
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) in asynchronous HeLa cells showing cytoplasmic and nuclear localization. Images shown are without (left panel) or with (right panel) nuclear Hoechst staining. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 15 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) at a dilution of 1:50 for at least 1 hour at room temperature, washed with PBS, and incubated with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502). Nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249) for 30 minutes. Images were taken on a Thermo Scientific ToxInsight Instrument at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
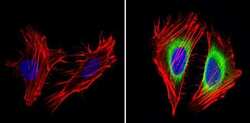
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) showing staining in the in the cytoplasm of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
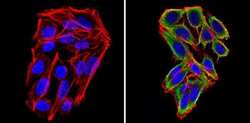
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) showing staining in the in the cytoplasm of SW480 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
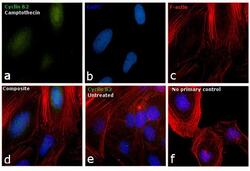
- Experimental details
- Immunofluorescence analysis of Cyclin B2 was performed using 70% confluent log phase HeLa cells treated with Camptothecin (100nM, 16 hrs). The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Cyclin B2 Monoclonal Antibody (Product # MA1-156) at 1:100 dilution in 0.1% BSA, incubated at 4 degree Celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing accumulation of Cyclin A2 in the nucleus upon treatment. Panel e represents the control cells showing cytoplasmic staining. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
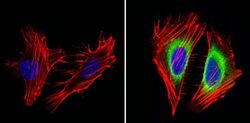
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) showing staining in the in the cytoplasm of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
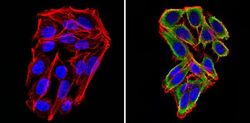
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) showing staining in the in the cytoplasm of SW480 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
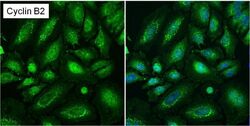
- Experimental details
- Immunofluorescent analysis of Cyclin B2 (green) in asynchronous HeLa cells showing cytoplasmic and nuclear localization. Images shown are without (left panel) or with (right panel) nuclear Hoechst staining. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 15 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) at a dilution of 1:50 for at least 1 hour at room temperature, washed with PBS, and incubated with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502). Nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249) for 30 minutes. Images were taken on a Thermo Scientific ToxInsight Instrument at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
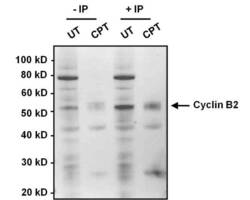
- Experimental details
- Immunoprecipitation of Cyclin B2 was performed on HeLa cell lysates, from cells left untreated (UT) or treated with 100 nM camptothecin (CPT). Antigen-antibody complexes were formed by incubating 375 µg of lysate with 1 µg of a Cyclin B2 monoclonal antibody (Product # MA1-156) overnight on an end-over-end rotator at 4°C. The immune complexes were captured on 100 µL Protein A/G Plus Agarose (Product # 20423), washed extensively, and eluted with 5X Lane Marker Reducing Sample Buffer (Product # 39000). Samples, including input cell lysate as a positive control, were resolved on a 4-12% Bis-Tris polyacrylamide gel, transferred to a nitrocellulose membrane and blocked with 5% BSA/TBST for 1 hour. The membrane was probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) at a dilution of 1:1000 overnight rotating at 4°C, washed in TBST, and probed with Clean-Blot IP Detection Reagent (Product # 21230). Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34076).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
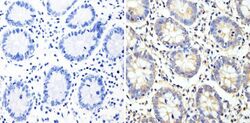
- Experimental details
- Immunohistochemistry analysis of Cyclin B2 showing staining in the cytoplasm of paraffin-embedded human colon tissue (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
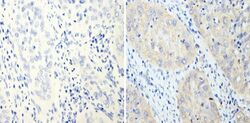
- Experimental details
- Immunohistochemistry analysis of Cyclin B2 showing staining in the cytoplasm of paraffin-embedded human cervical carcinoma (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) diluted in 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
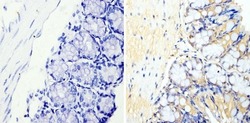
- Experimental details
- Immunohistochemistry analysis of Cyclin B2 showing staining in the nucleus and cytoplasm of paraffin-embedded mouse colon tissue (right) compared with a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) diluted in 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
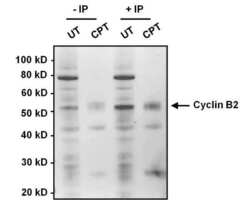
- Experimental details
- Immunoprecipitation of Cyclin B2 was performed on HeLa cell lysates, from cells left untreated (UT) or treated with 100 nM camptothecin (CPT). Antigen-antibody complexes were formed by incubating 375 µg of lysate with 1 µg of a Cyclin B2 monoclonal antibody (Product # MA1-156) overnight on an end-over-end rotator at 4øC. The immune complexes were captured on 100 µL Protein A/G Plus Agarose (Product # 20423), washed extensively, and eluted with 5X Lane Marker Reducing Sample Buffer (Product # 39000). Samples, including input cell lysate as a positive control, were resolved on a 4-12% Bis-Tris polyacrylamide gel, transferred to a nitrocellulose membrane and blocked with 5% BSA/TBST for 1 hour. The membrane was probed with a Cyclin B2 monoclonal antibody (Product # MA1-156) at a dilution of 1:1000 overnight rotating at 4øC, washed in TBST, and probed with Clean-Blot IP Detection Reagent (Product # 21230). Chemiluminescent detection was performed using SuperSignal West Dura (Product # 34076).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry