PA3-850
antibody from Invitrogen Antibodies
Targeting: IGF2R
CD222, CI-M6PR, CI-MPR, CIMPR, M6P-R, MPR1, MPR300, MPRI
Antibody data
- Antibody Data
- Antigen structure
- References [5]
- Comments [0]
- Validations
- Immunocytochemistry [3]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA3-850 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- IGF2R Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Purifed from natural sources
- Description
- PA3-850 detects cation-independent mannose-6-receptor(CI-MPR) in human, bovine, mouse and non-human primate cells. PA3-850 has been successfully used in ICC/IF, Dot blot and Western blotting procedures. By Western blot, it detects a 300 kDa protein representing CI-MPR under non-reducing conditions. The PA3-850 immunogen is a 300 kDa cation-independent receptor purified from adult Bovine liver tissue.
- Reactivity
- Human, Mouse, Bovine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 µL
- Concentration
- Conc. Not Determined
- Storage
- -20° C, Avoid Freeze/Thaw Cycles
Submitted references Tumor protein D54 defines a new class of intracellular transport vesicles.
Chlamydia trachomatis CT229 Subverts Rab GTPase-Dependent CCV Trafficking Pathways to Promote Chlamydial Infection.
Arf6 controls retromer traffic and intracellular cholesterol distribution via a phosphoinositide-based mechanism.
Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing.
Identification of novel murine- and human-specific RPGRIP1 splice variants with distinct expression profiles and subcellular localization.
Larocque G, La-Borde PJ, Clarke NI, Carter NJ, Royle SJ
The Journal of cell biology 2020 Jan 6;219(1)
The Journal of cell biology 2020 Jan 6;219(1)
Chlamydia trachomatis CT229 Subverts Rab GTPase-Dependent CCV Trafficking Pathways to Promote Chlamydial Infection.
Faris R, Merling M, Andersen SE, Dooley CA, Hackstadt T, Weber MM
Cell reports 2019 Mar 19;26(12):3380-3390.e5
Cell reports 2019 Mar 19;26(12):3380-3390.e5
Arf6 controls retromer traffic and intracellular cholesterol distribution via a phosphoinositide-based mechanism.
Marquer C, Tian H, Yi J, Bastien J, Dall'Armi C, Yang-Klingler Y, Zhou B, Chan RB, Di Paolo G
Nature communications 2016 Jun 23;7:11919
Nature communications 2016 Jun 23;7:11919
Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing.
Bohnsack RN, Warejcka DJ, Wang L, Gillespie SR, Bernstein AM, Twining SS, Dahms NM
Investigative ophthalmology & visual science 2014 Oct 30;55(12):7697-708
Investigative ophthalmology & visual science 2014 Oct 30;55(12):7697-708
Identification of novel murine- and human-specific RPGRIP1 splice variants with distinct expression profiles and subcellular localization.
Lu X, Ferreira PA
Investigative ophthalmology & visual science 2005 Jun;46(6):1882-90
Investigative ophthalmology & visual science 2005 Jun;46(6):1882-90
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
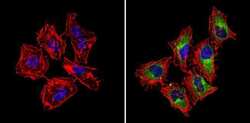
- Experimental details
- Immunofluorescent analysis of CI-MPR (green) showing staining in the cytoplasm of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CI-MPR polyclonal antibody (Product # PA3-850) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
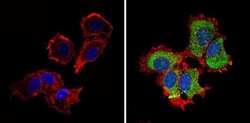
- Experimental details
- Immunofluorescent analysis of CI-MPR (green) showing staining in the cytoplasm and nucleus of MCF-7 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CI-MPR polyclonal antibody (Product # PA3-850) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
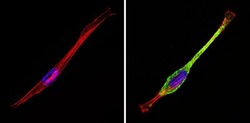
- Experimental details
- Immunofluorescent analysis of CI-MPR (green) showing staining in the cytoplasm of NIH-3T3 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CI-MPR polyclonal antibody (Product # PA3-850) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
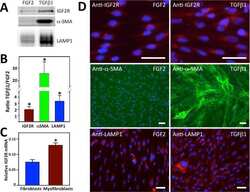
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
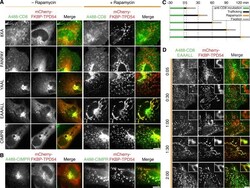
- Experimental details
- Figure 7. TPD54 co-reroutes dileucine motif-containing receptors only. (A) Representative widefield micrographs of cells coexpressing mCherry-FKBP-TPD54, dark MitoTrap, and the indicated CD8 construct. Rerouting was induced by 200 nM rapamycin. Cells were fixed, permeabilized, and stained for total CD8. (B) Representative widefield micrographs showing co-rerouting of endogenous CIMPR detected by immunofluorescence with rerouting of mCherry-FKBP-TPD54 to dark MitoTrap by addition of 200 nM rapamycin. (C) Pulse label and timed vesicle capture experiments. Cells expressing CD8-EAAALL were surface labeled with Alexa Fluor 488-conjugated anti-CD8 antibodies for 30 min, then incubated at 37degC for the indicated time (minutes), treated with 200 nM rapamycin for 5 min, and fixed. (D) Representative widefield micrographs from a pulse label and timed vesicle capture experiment. Inset, 5x zoom. Scale bars, 10 um, 1 um (insets). Time, hours and minutes.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry