Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunocytochemistry [1]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 702831 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Cdc5L Recombinant Rabbit Monoclonal Antibody (22H12L1)
- Antibody type
- Monoclonal
- Antigen
- Other
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Antibody clone number
- 22H12L1
- Vial size
- 100 µg
- Concentration
- 0.5 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Cell Division Cycle 5-Like Regulates Metaphase-to-Anaphase Transition in Meiotic Oocyte.
Zhang HY, Li J, Ouyang YC, Meng TG, Zhang CH, Yue W, Sun QY, Qian WP
Frontiers in cell and developmental biology 2021;9:671685
Frontiers in cell and developmental biology 2021;9:671685
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
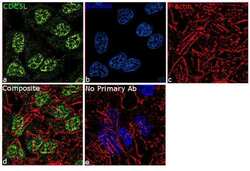
- Experimental details
- For immunofluorescence analysis,HeLa cells were fixed and permeabilized for detection of endogenous CDC5L using Anti-CDC5L Recombinant Rabbit Monoclonal Antibody (Product # 702831, 1:100) and labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034, 1:2000).Panel a) shows representative cells that were stained for detection and localization of CDC5L protein (green), panel b is stained for nuclei (blue) using SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). Panel c) represents cytoskeletal F-actin staining using Rhodamine Phalloidin (Product # R415, 1:300). Panel d) is a composite image of Panels a, b and c demonstrating predominant nuclear localization of CDC5L and panel e) represents control cells without primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
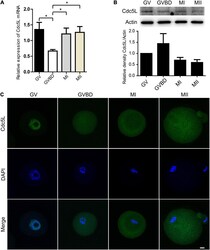
- Experimental details
- FIGURE 1 Expression and subcellular localization of Cdc5L during mouse oocyte meiotic maturation. (A) Relative level of Cdc5L mRNA identified by RT-PCR. A total of 50 oocytes were collected for each sample. At least three replications were conducted, and 150 oocytes were used for each group. Data are shown as means +- SEM. ""*"" represents P < 0.05; ""**"" represents P < 0.01. (B) Expression of Cdc5L protein revealed by Western blotting analysis. A total of 150 oocytes were collected after culture for 0, 4, 8, and 12 h; time points when most oocytes had reached GV, GVBD, MI, and MII stages, respectively. At least three replications were conducted, and 450 oocytes were used for each group. beta-actin is shown as an internal control. (C) Subcellular localization of Cdc5L as revealed by immunofluorescence staining. Confocal microscopy images that showed subcellular localization of Cdc5L (green) and DNA (blue) was counterstained with DAPI. Scale bars: 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
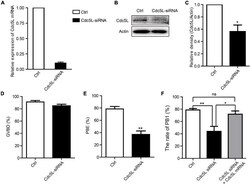
- Experimental details
- FIGURE 2 Knockdown of Cdc5L reduces rate of PB1 extrusion. (A) Relative level of Cdc5L mRNA identified by RT-PCR in control and Cdc5L siRNA oocytes. A total of 50 oocytes were collected in each group after culture for 24 h in M2 medium with IBMX. At least three replications were conducted, and 150 oocytes were used for each group. Data are shown as means +- SEM. ""*"" represents P < 0.05; ""**"" represents P < 0.01. (B) Western blotting results for Cdc5L and beta-actin in Cdc5L-siRNA injected oocytes and control oocytes after culture for 24 h in M2 medium with IBMX (150 oocytes per sample). At least three replications were conducted, and 450 oocytes were used for each group. (C) Relative staining intensity of Cdc5L assessed by densitometry. Data are shown as means +- SEM. ""*"" represents P < 0.05; ""**"" represents P < 0.01. At least three replications were conducted. (D) Percentages of GVBD were quantified in control and Cdc5L siRNA oocytes. Data are shown as means +- SEM. ""*"" represents P < 0.05; ""**"" represents P < 0.01. At least three replications were conducted. (E) Rate of PB1 extrusion was quantified in control and Cdc5L siRNA oocytes. Data are shown as means +- SEM. ""*"" represents P < 0.05; ""**"" represents P < 0.01. At least three replications were conducted. (F) Rates of PB1 extrusion were quantified in control, Cdc5L siRNA, and Cdc5L mRNA + siRNA oocytes. Data are shown as means +- SEM. ""*"" represents P < 0.05; ""**"" represents P < 0.01. At least three r
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry