Antibody data
- Antibody Data
- Antigen structure
- References [19]
- Comments [0]
- Validations
- Immunocytochemistry [5]
- Immunohistochemistry [3]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-12272 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- alpha Synuclein Monoclonal Antibody (Syn 211)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- MA5-12272 targets Synuclein in IHC (P), ICC/IF, IP, and WB applications and shows reactivity with Human and Rat samples. The MA5-12272 immunogen is human recombinant alpha-synuclein.
- Reactivity
- Human, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- Syn 211
- Vial size
- 500 μL
- Concentration
- 0.2 mg/mL
- Storage
- 4°C
Submitted references Angiotensin-(1-7) reduces α-synuclein aggregation by enhancing autophagic activity in Parkinson's disease.
Cofilin 1 promotes the pathogenicity and transmission of pathological α-synuclein in mouse models of Parkinson's disease.
Alpha-Synuclein in the Regulation of Brain Endothelial and Perivascular Cells: Gaps and Future Perspectives.
Alpha-synuclein is involved in manganese-induced spatial memory and synaptic plasticity impairments via TrkB/Akt/Fyn-mediated phosphorylation of NMDA receptors.
Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson's Disease: An In Situ Proximity Ligation Assay Study.
Lack of pathogenic potential of peripheral α-synuclein aggregates from Parkinson's disease patients.
Cortical phosphorylated α-Synuclein levels correlate with brain wave spectra in Parkinson's disease.
A brain-targeted, modified neurosin (kallikrein-6) reduces α-synuclein accumulation in a mouse model of multiple system atrophy.
Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by α-synuclein overexpression.
Neurite Aggregation and Calcium Dysfunction in iPSC-Derived Sensory Neurons with Parkinson's Disease-Related LRRK2 G2019S Mutation.
Hypoestoxide reduces neuroinflammation and α-synuclein accumulation in a mouse model of Parkinson's disease.
Differential expression of alpha-synuclein in hippocampal neurons.
α-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity.
α-synuclein phosphorylation and truncation are normal events in the adult human brain.
Neuropathology in mice expressing mouse alpha-synuclein.
Alpha-synuclein mediates alterations in membrane conductance: a potential role for alpha-synuclein oligomers in cell vulnerability.
Identification of glutathione S-transferase pi as a protein involved in Parkinson disease progression.
A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3.
Abnormal alpha-synuclein solubility, aggregation and nitration in the frontal cortex in Pick's disease.
Gao Q, Chen R, Wu L, Huang Q, Wang XX, Tian YY, Zhang YD
Neural regeneration research 2022 May;17(5):1138-1145
Neural regeneration research 2022 May;17(5):1138-1145
Cofilin 1 promotes the pathogenicity and transmission of pathological α-synuclein in mouse models of Parkinson's disease.
Yan M, Xiong M, Dai L, Zhang X, Zha Y, Deng X, Yu Z, Zhang Z
NPJ Parkinson's disease 2022 Jan 10;8(1):1
NPJ Parkinson's disease 2022 Jan 10;8(1):1
Alpha-Synuclein in the Regulation of Brain Endothelial and Perivascular Cells: Gaps and Future Perspectives.
Bogale TA, Faustini G, Longhena F, Mitola S, Pizzi M, Bellucci A
Frontiers in immunology 2021;12:611761
Frontiers in immunology 2021;12:611761
Alpha-synuclein is involved in manganese-induced spatial memory and synaptic plasticity impairments via TrkB/Akt/Fyn-mediated phosphorylation of NMDA receptors.
Ma Z, Liu K, Li XR, Wang C, Liu C, Yan DY, Deng Y, Liu W, Xu B
Cell death & disease 2020 Oct 8;11(10):834
Cell death & disease 2020 Oct 8;11(10):834
Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson's Disease: An In Situ Proximity Ligation Assay Study.
Longhena F, Faustini G, Missale C, Pizzi M, Bellucci A
International journal of molecular sciences 2018 May 30;19(6)
International journal of molecular sciences 2018 May 30;19(6)
Lack of pathogenic potential of peripheral α-synuclein aggregates from Parkinson's disease patients.
Recasens A, Carballo-Carbajal I, Parent A, Bové J, Gelpi E, Tolosa E, Vila M
Acta neuropathologica communications 2018 Feb 8;6(1):8
Acta neuropathologica communications 2018 Feb 8;6(1):8
Cortical phosphorylated α-Synuclein levels correlate with brain wave spectra in Parkinson's disease.
Caviness JN, Lue LF, Hentz JG, Schmitz CT, Adler CH, Shill HA, Sabbagh MN, Beach TG, Walker DG
Movement disorders : official journal of the Movement Disorder Society 2016 Jul;31(7):1012-9
Movement disorders : official journal of the Movement Disorder Society 2016 Jul;31(7):1012-9
A brain-targeted, modified neurosin (kallikrein-6) reduces α-synuclein accumulation in a mouse model of multiple system atrophy.
Spencer B, Valera E, Rockenstein E, Trejo-Morales M, Adame A, Masliah E
Molecular neurodegeneration 2015 Sep 23;10:48
Molecular neurodegeneration 2015 Sep 23;10:48
Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by α-synuclein overexpression.
Bourdenx M, Dovero S, Engeln M, Bido S, Bastide MF, Dutheil N, Vollenweider I, Baud L, Piron C, Grouthier V, Boraud T, Porras G, Li Q, Baekelandt V, Scheller D, Michel A, Fernagut PO, Georges F, Courtine G, Bezard E, Dehay B
Acta neuropathologica communications 2015 Jul 25;3:46
Acta neuropathologica communications 2015 Jul 25;3:46
Neurite Aggregation and Calcium Dysfunction in iPSC-Derived Sensory Neurons with Parkinson's Disease-Related LRRK2 G2019S Mutation.
Schwab AJ, Ebert AD
Stem cell reports 2015 Dec 8;5(6):1039-1052
Stem cell reports 2015 Dec 8;5(6):1039-1052
Hypoestoxide reduces neuroinflammation and α-synuclein accumulation in a mouse model of Parkinson's disease.
Kim C, Ojo-Amaize E, Spencer B, Rockenstein E, Mante M, Desplats P, Wrasidlo W, Adame A, Nchekwube E, Oyemade O, Okogun J, Chan M, Cottam H, Masliah E
Journal of neuroinflammation 2015 Dec 18;12:236
Journal of neuroinflammation 2015 Dec 18;12:236
Differential expression of alpha-synuclein in hippocampal neurons.
Taguchi K, Watanabe Y, Tsujimura A, Tatebe H, Miyata S, Tokuda T, Mizuno T, Tanaka M
PloS one 2014;9(2):e89327
PloS one 2014;9(2):e89327
α-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity.
Chen RHC, Wislet-Gendebien S, Samuel F, Visanji NP, Zhang G, Marsilio D, Langman T, Fraser PE, Tandon A
The Journal of biological chemistry 2013 Mar 15;288(11):7438-7449
The Journal of biological chemistry 2013 Mar 15;288(11):7438-7449
α-synuclein phosphorylation and truncation are normal events in the adult human brain.
Muntané G, Ferrer I, Martinez-Vicente M
Neuroscience 2012 Jan 3;200:106-19
Neuroscience 2012 Jan 3;200:106-19
Neuropathology in mice expressing mouse alpha-synuclein.
Rieker C, Dev KK, Lehnhoff K, Barbieri S, Ksiazek I, Kauffmann S, Danner S, Schell H, Boden C, Ruegg MA, Kahle PJ, van der Putten H, Shimshek DR
PloS one 2011;6(9):e24834
PloS one 2011;6(9):e24834
Alpha-synuclein mediates alterations in membrane conductance: a potential role for alpha-synuclein oligomers in cell vulnerability.
Feng LR, Federoff HJ, Vicini S, Maguire-Zeiss KA
The European journal of neuroscience 2010 Jul;32(1):10-7
The European journal of neuroscience 2010 Jul;32(1):10-7
Identification of glutathione S-transferase pi as a protein involved in Parkinson disease progression.
Shi M, Bradner J, Bammler TK, Eaton DL, Zhang J, Ye Z, Wilson AM, Montine TJ, Pan C, Zhang J
The American journal of pathology 2009 Jul;175(1):54-65
The American journal of pathology 2009 Jul;175(1):54-65
A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3.
Holm IE, Englund E, Mackenzie IR, Johannsen P, Isaacs AM
Journal of neuropathology and experimental neurology 2007 Oct;66(10):884-91
Journal of neuropathology and experimental neurology 2007 Oct;66(10):884-91
Abnormal alpha-synuclein solubility, aggregation and nitration in the frontal cortex in Pick's disease.
Dalfó E, Martinez A, Muntané G, Ferrer I
Neuroscience letters 2006 May 29;400(1-2):125-9
Neuroscience letters 2006 May 29;400(1-2):125-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
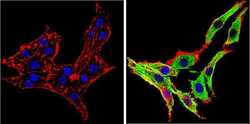
- Experimental details
- Immunofluorescent analysis of Synuclein (green) showing staining in the cytoplasm of PC12 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Synuclein monoclonal antibody (Product # MA5-12272) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
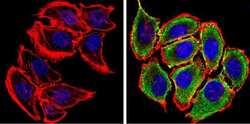
- Experimental details
- Immunofluorescent analysis of Synuclein (green) showing staining in the cytoplasm and membrane of U251 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Synuclein monoclonal antibody (Product # MA5-12272) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
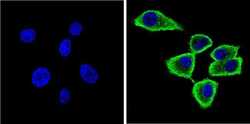
- Experimental details
- Immunofluorescent analysis of Synuclein (green) showing staining in the cytoplasm and membrane of U87-MG cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Synuclein monoclonal antibody (Product # MA5-12272) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
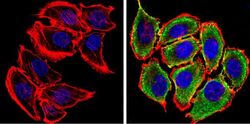
- Experimental details
- Immunofluorescent analysis of Synuclein (green) showing staining in the cytoplasm and membrane of U251 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Synuclein monoclonal antibody (Product # MA5-12272) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
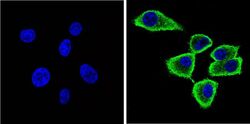
- Experimental details
- Immunofluorescent analysis of Synuclein (green) showing staining in the cytoplasm and membrane of U87-MG cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Synuclein monoclonal antibody (Product # MA5-12272) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
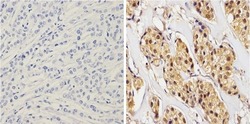
- Experimental details
- Immunohistochemistry analysis of Synuclein showing positive staining in the cytoplasm and nucleus of paraffin-treated Human breast carcinoma (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Synuclein monoclonal antibody (Product # MA5-12272) diluted by 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
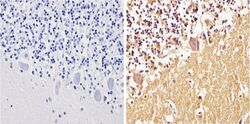
- Experimental details
- Immunohistochemistry analysis of Synuclein showing positive staining in the cytoplasm and nucleus of paraffin-treated Human cerebellum tissue (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Synuclein monoclonal antibody (Product # MA5-12272) diluted by 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
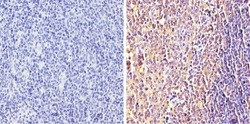
- Experimental details
- Immunohistochemistry analysis of Synuclein showing positive staining in the cytoplasm and nucleus of paraffin-treated Human tonsil tissue (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Synuclein monoclonal antibody (Product # MA5-12272) diluted by 3% BSA-PBS at a dilution of 1:100 overnight at 4°C in a humidified chamber. Tissues were washed extensively PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
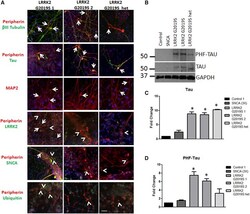
- Figure 3 Aggregates Are Comprised of Cytoskeletal Proteins (A) LRRK2 G2019S aggregates were positive (indicated by arrows) for betaIII tubulin (green) and peripherin (red), tau (green), MAP2 (red), and LRRK2 (green). Aggregates were generally negative (indicated by arrowheads) for SNCA and ubiquitin (green). (B-D) LRRK2 G2019S sensory neuron cultures display significantly increased levels of (B and C) tau and (B and D) phospho-tau by western blot and densitometry. * p < 0.01 by ANOVA; n = 6 independent experiments. The scale bar represents 50 mum. See also Figure S3 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
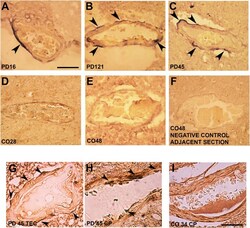
- Figure 3 Alpha-synuclein perivascular immunoreactivity in postmortem sections from sporadic patients with PD. For these experiments, sections from three patients with PD (PD16, disease duration 18 years; PD45, disease duration 19 years; and PD121, disease duration 4 years) and three healthy controls (CO28, CO34, and CO48), kindly supplied by the Parkinson's UK Brain Bank, were analyzed. Briefly, sections were treated for antigen retrieval with 10 mM sodium citrate (20' at 95degC) and 10% formic acid (15' at RT). After 1 h incubation at room temperature (RT) with blocking solution (2% w/vol bovine serum albumin, 3% vol/vol normal goat serum, 0.3% Triton X-100 diluted in PBS 0.1 M pH 7.4) the 5-mum slices were subjected to either single alpha-synuclein (Sin211 MA5-12272 Thermo Fisher Scientific, Waltham, USA; dilution 1:500) or double laminin alpha2 (4H8-2, abcam ab11576; dilution 1:100)/alpha-synuclein (Sin211, MA5-12272 Thermo Fisher; dilution 1:500) immunolabeling according to previously described protocols (, ). Single alpha-synuclein immunopositive signal was revealed by Blue Alkaline Phosphatase (Vector Laboratories, Burlingame, CA) acquired by using a 40X objective, while for double immunolabeling laminin alpha2 was revealed by brown 3,3-diaminobenzidine (DAB) and alpha-synuclein by violet (Nickel supplemented) DAB (Vector laboratories) and acquired by a 100X objective. All the images were acquired by using an inverted light microscope (Olympus BX41; Olympus, Milan, Ital
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
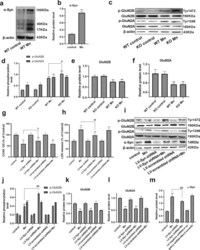
- Fig. 3 Mn-dependent enhanced alpha-Syn expression disturbs the phosphorylation of NMDA receptors in vivo and in vitro. a , b After treatment with Mn, western blotting was used to measure the level of alpha-Syn overexpression in the hippocampus. c - f The levels of phosphorylated NMDA receptors (GluN2B and GluN2A) were measured by western blotting in vivo. n = 6. g , h HT22 cells were transfected with LV-alpha-Syn shRNA, and the cytotoxicity was measured using the CCK-8 assay and lactate dehydrogenase (LDH) release assay. i - m The levels of phosphorylated NMDA receptors (GluN2B and GluN2A) and alpha-Syn expression were measured by western blotting in vitro. n = 4. ** P < 0.01 compared to their control counterparts; # # P < 0.01, and # P < 0.05 for comparison between the Mn treatment group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
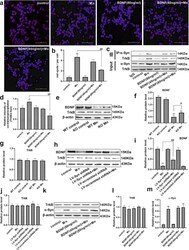
- Fig. 4 Brain-derived neurotrophic factor (BDNF) interfered with the interaction of alpha-Syn and TrkB. a , b After pretreatment with BDNF and Mn in HT22 cells, representative images of PLA show the interaction between alpha-Syn and TrkB (red spots), and the nucleus was stained in blue (DAPI). Scale bars = 50 mum. c , d The immunoprecipitation analysis of alpha-Syn and TrkB is shown. e - g After treating the mice with Mn, the levels of BDNF and TrkB were evaluated by western blotting. n = 6. h - j For HT22 cells transfected with the LV-alpha-Syn shRNA, the levels of BDNF and TrkB were evaluated by western blotting after Mn treatment. k - m After pretreatment with BDNF and Mn in HT22 cells, the levels of TrkB and alpha-Syn expression were evaluated by western blotting. n = 4. ** P < 0.01, and * P < 0.05 compared to their control counterparts; ## P < 0.01, and # P < 0.05 for comparison between the Mn treatment group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
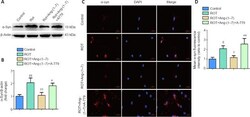
- Figure 5 Ang-(1-7) attenuates alpha-synuclein accumulation in the rotenone-induced cell model in a MasR-dependent manner . (A) The expression of alpha-syn in four groups was examined by Western blot assay. (B) Quantitative results of alpha-syn expression. (C) Cells were marked by an anti-alpha-syn antibody (red), nuclei were counterstained with DAPI (blue), and immunofluorescence was observed by fluorescent microscopy (original magnification, 630x). Expression of alpha-syn was higher in the ROT group and was downregulated when incubated with Ang-(1-7). However, the influence caused by Ang-(1-7) was completely abolished with A-779 co-treatment. (D) Mean alpha-syn fluorescence intensity (ratio to control) of four groups. Data are expressed as the mean the +- SD ( n = 3; one-way analysis of variance followed by Tukey's post hoc test). # P < 0.05, ## P < 0.01, vs . control group; + P < 0.05, ++ P < 0.01, vs . ROT group; * P < 0.05, ** P < 0.01, vs . ROT + Ang-(1-7) group. Ang-(1-7): Angiotensin-(1-7); DAPI: 4',6-diamidino-2-phenylindole; MasR: Mas receptor; ROT: rotenone; alpha-syn: alpha-synuclein.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
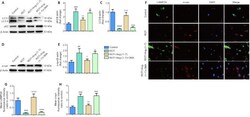
- Figure 6 Activation of autophagy contributes to the clearance of alpha-synuclein accumulation in the rotenone-induced cell model . (A)The p62 and LC3-II levels were determined by Western blot assay. (B) Quantitative results of p62 expression. (C) Quantitative results of LC3-II expression. (D) The expression of alpha-syn was detected by Western blot assay. (E) Quantitative results of alpha-syn expression. (F) The number of LAMP2A-positive cells (green) and alpha-syn-positive cells (red) by immunofluorescence were observed with a fluorescent microscope (original magnification, 630x). (G) Mean LAMP2A fluorescent intensity (ratio to control) of four groups. (H) Mean alpha-syn fluorescence intensity (ratio to control) of four groups. Data are expressed as the mean +- SD (B, C, E; n = 3; one-way analysis of variance followed by Tukey's post hoc test). ## P < 0.01, ### P < 0.001, #### P < 0.0001, vs . control group; + P < 0.05, ++ P < 0.01, +++ P < 0.001, ++++ P < 0.0001, vs . ROT group; * P < 0.05, *** P < 0.001, **** P < 0.0001, vs . ROT + Ang-(1-7) group. 3-MA: 3-Methyladenine; Ang-(1-7): angiotensin-(1-7); DAPI: 4',6-diamidino-2-phenylindole; LAMP2A: lysosomal-associated membrane protein 2A; LC3: microtubule associated protein 1 light chain 3; ROT: rotenone; alpha-syn: alpha-synuclein.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
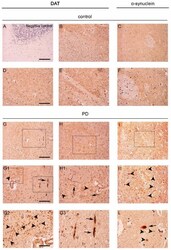
- Figure 1 Dopamine transporter (DAT) staining in the caudate putamen of PD patients and age-matched controls. ( A ) Representative photomicrograph showing the negative control produced by performing DAT immunolabeling on cerebellum sections. Please note the absence of staining that is indicative of the specificity of the DAT antibody used for this study; ( B , D , E , G , H , G1 , H1 , G2 , G3 ) Representative images showing DAT immunolabeling in the caudate putamen of PD patients and age-matched controls subjects. DAT immunolabeling showed a small dot-like appearance with widespread distribution in the control sections ( B , D , E ). Please note that in spite of the overall decrease of immunolabeling, in the PD brains, the DAT signal was accumulated in big neuropil-like dots ( G , H , G1 , H1 , G2 , arrowheads) and in few neuritic-like structures ( G , G1 , G3 , arrows); ( C , F , I , I1 , L ) Panels are showing alpha-synuclein immunolabeling in the caudate putamen of PD patients and age-matched control subjects. Alpha-synuclein immunolabeling showed a widespread distribution in the grey matter in control sections ( C , F ). Please note the presence of neuropil-like alpha-synuclein-positive dots ( I , I1 , L , arrowheads) and LB-like structures in the PD brains ( L , arrow). Scale bars: ( A - C , G - I ) 100 um, ( D - F , G1 , H1 , I1 ) 50 um, ( G2 , G3 , L ) 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
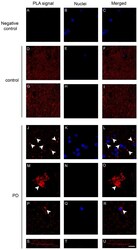
- Figure 4 Dopamine transporter (DAT)/alpha-synuclein in situ PLA performed on the caudate putamen of PD patients and control subjects. ( A - C ) Panels showing the negative control, performed by omitting the DAT antibody, of the in situ PLA assayed on the caudate putamen of a control subject. The absence of the PLA-positive signal is indicative of the specificity of the assay; ( D - U ) Representative images showing DAT/alpha-synuclein in situ PLA performed on the caudate putamen of PD patients ( J - U ) and age-matched-controls (control, D - I ). The presence of a PLA positivity as a red fluorescent signal is indicative of the interaction between the two proteins. Please note the marked redistribution of the PLA-positivity in the caudate putamen of PD samples ( L , O , R ) when compared to the control subjects ( F , I ). In particular, in the PD brains, the PLA signal was particularly abundant within big ( O , arrowheads) and small ( L , R , arrowheads) clumps or neuritic-like structures ( U ). Scale bar: 20 mum.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation