Antibody data
- Antibody Data
- Antigen structure
- References [9]
- Comments [0]
- Validations
- Immunocytochemistry [4]
- Immunohistochemistry [1]
- Flow cytometry [1]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 701085 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- alpha Synuclein Recombinant Rabbit Monoclonal Antibody (14H2L1)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- Intact IgG appears on a non-reducing gel as ~150 kDa band and upon reduction generating a ~25 kDa light chain band and a ~50 kDa heavy chain. Recombinant rabbit monoclonal antibodies are produced using in vitro expression systems. The expression systems are developed by cloning in the specific antibody DNA sequences from immunoreactive rabbits. Then, individual clones are screened to select the best candidates for production. The advantages of using recombinant rabbit monoclonal antibodies include: better specificity and sensitivity, lot-to-lot consistency, animal origin-free formulations, and broader immunoreactivity to diverse targets due to larger rabbit immune repertoire.
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Antibody clone number
- 14H2L1
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Exposure of α-Synuclein Aggregates to Organotypic Slice Cultures Recapitulates Key Molecular Features of Parkinson's Disease.
Development of a physiologically relevant and easily scalable LUHMES cell-based model of G2019S LRRK2-driven Parkinson's disease.
Altered Gut Microbiome in Parkinson's Disease and the Influence of Lipopolysaccharide in a Human α-Synuclein Over-Expressing Mouse Model.
Inhibition of nSMase2 Reduces the Transfer of Oligomeric α-Synuclein Irrespective of Hypoxia.
DNA repair deficiency and senescence in concussed professional athletes involved in contact sports.
DNA damage as a marker of brain damage in individuals with history of concussions.
Protective efficacy of phosphodiesterase-1 inhibition against alpha-synuclein toxicity revealed by compound screening in LUHMES cells.
Nonaggregated α-synuclein influences SNARE-dependent vesicle docking via membrane binding.
α-Synuclein as an intrinsically disordered monomer--fact or artefact?
Moudio S, Rodin F, Albargothy NJ, Karlsson U, Reyes JF, Hallbeck M
Frontiers in neurology 2022;13:826102
Frontiers in neurology 2022;13:826102
Development of a physiologically relevant and easily scalable LUHMES cell-based model of G2019S LRRK2-driven Parkinson's disease.
Calamini B, Geyer N, Huss-Braun N, Bernhardt A, Harsany V, Rival P, Cindhuchao M, Hoffmann D, Gratzer S
Disease models & mechanisms 2021 Jun 1;14(6)
Disease models & mechanisms 2021 Jun 1;14(6)
Altered Gut Microbiome in Parkinson's Disease and the Influence of Lipopolysaccharide in a Human α-Synuclein Over-Expressing Mouse Model.
Gorecki AM, Preskey L, Bakeberg MC, Kenna JE, Gildenhuys C, MacDougall G, Dunlop SA, Mastaglia FL, Akkari PA, Koengten F, Anderton RS
Frontiers in neuroscience 2019;13:839
Frontiers in neuroscience 2019;13:839
Inhibition of nSMase2 Reduces the Transfer of Oligomeric α-Synuclein Irrespective of Hypoxia.
Sackmann V, Sinha MS, Sackmann C, Civitelli L, Bergström J, Ansell-Schultz A, Hallbeck M
Frontiers in molecular neuroscience 2019;12:200
Frontiers in molecular neuroscience 2019;12:200
DNA repair deficiency and senescence in concussed professional athletes involved in contact sports.
Schwab N, Grenier K, Hazrati LN
Acta neuropathologica communications 2019 Nov 14;7(1):182
Acta neuropathologica communications 2019 Nov 14;7(1):182
DNA damage as a marker of brain damage in individuals with history of concussions.
Schwab N, Tator C, Hazrati LN
Laboratory investigation; a journal of technical methods and pathology 2019 Jul;99(7):1008-1018
Laboratory investigation; a journal of technical methods and pathology 2019 Jul;99(7):1008-1018
Protective efficacy of phosphodiesterase-1 inhibition against alpha-synuclein toxicity revealed by compound screening in LUHMES cells.
Höllerhage M, Moebius C, Melms J, Chiu WH, Goebel JN, Chakroun T, Koeglsperger T, Oertel WH, Rösler TW, Bickle M, Höglinger GU
Scientific reports 2017 Sep 13;7(1):11469
Scientific reports 2017 Sep 13;7(1):11469
Nonaggregated α-synuclein influences SNARE-dependent vesicle docking via membrane binding.
Lai Y, Kim S, Varkey J, Lou X, Song JK, Diao J, Langen R, Shin YK
Biochemistry 2014 Jun 24;53(24):3889-96
Biochemistry 2014 Jun 24;53(24):3889-96
α-Synuclein as an intrinsically disordered monomer--fact or artefact?
Coelho-Cerqueira E, Carmo-Gonçalves P, Pinheiro AS, Cortines J, Follmer C
The FEBS journal 2013 Oct;280(19):4915-27
The FEBS journal 2013 Oct;280(19):4915-27
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
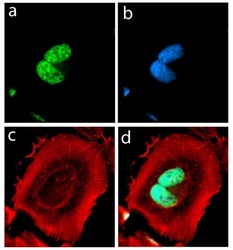
- Experimental details
- Immunofluorescent analysis of alpha Synuclein in HeLa cells using an Alpha Synuclein recombinant rabbit monoclonal antibody (Product # 701085) followed by detection using an Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (green) (Image A). Nuclei were stained using DAPI (Image B) and actin stained with Alexa Fluor 594 phalloidin (red) (image C). Image D is a composite image showing cytoplasmic and nuclear localization of Alpha-Synuclein.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
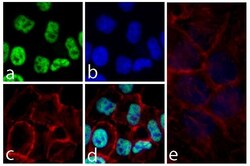
- Experimental details
- Immunofluorescent analysis of SNCA was performed on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0. 25% Triton X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with SNCA Recombinant Rabbit Monoclonal Antibody (Product # 701085) at a dilution of 1:1000 in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor® 488 Goat anti-Rabbit IgG secondary antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 594 phalloidin (Product # A12381) and Panel d is a merged image showing nuclear localization and panel e is a control without primary antibody. The images were captured using a Nikon microscope at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
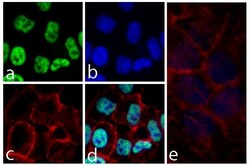
- Experimental details
- Immunofluorescent analysis of SNCA was performed on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0. 25% Triton X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with SNCA Recombinant Rabbit Monoclonal Antibody (Product # 701085) at a dilution of 1:1000 in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Fluor® 488 Goat anti-Rabbit IgG secondary antibody (Product # A-11008) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 594 phalloidin (Product # A12381) and Panel d is a merged image showing nuclear localization and panel e is a control without primary antibody. The images were captured using a Nikon microscope at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
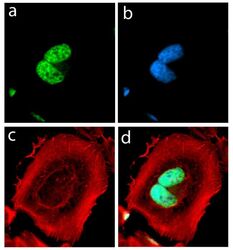
- Experimental details
- Immunofluorescent analysis of alpha Synuclein in HeLa cells using an Alpha Synuclein recombinant rabbit monoclonal antibody (Product # 701085) followed by detection using an Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (green) (Image A). Nuclei were stained using DAPI (Image B) and actin stained with Alexa Fluor 594 phalloidin (red) (image C). Image D is a composite image showing cytoplasmic and nuclear localization of Alpha-Synuclein.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
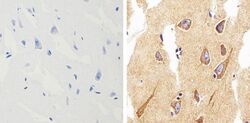
- Experimental details
- Immunohistochemistry analysis of alpha Synuclein showing staining in the cytoplasm of paraffin-embedded human brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10 mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with alpha Synuclein monoclonal antibody (Product # 701085) diluted in 3% BSA-PBS at a dilution of 1:500 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using a HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
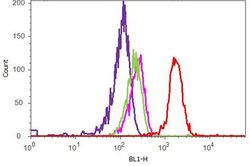
- Experimental details
- Flow cytometry analysis of SNCA was performed on HeLa cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0. 25% Tritonª X-100 for 20 minutes, and blocked with 5% BSA for 1 hour at room temperature. Cells were labeled with ABfinityª SNCA recombinant rabbit monoclonal antibody (Product # 701085, red histogram) or with rabbit isotype control (pink histogram) at a dilution of 1:400 in 2.5% BSA. After incubation at room temperature for 3 hours, the cells were labeled with Alexa Fluor¨ 488 goat anti-Rabbit Secondary antibody (Product # A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune¨ Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
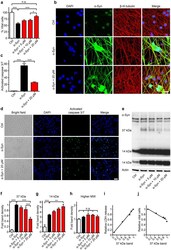
- Experimental details
- Figure 3 The PDE1 inhibitor vinpocetine reduces cell death and a 37 kDa alpha-Syn species. ( a ) Counts of vital, i.e. non-clumped, non-fragmented 4',6-diamidino-2-phenylindole (DAPI)-stained nuclei in untransduced control neurons (Ctrl, white bar), alpha-Syn transduced neurons (black bar) treated with solvent only, and alpha-Syn transduced neurons treated with different concentrations of vinpocetine (red bars) confirmed the compound's protective efficacy. ( b ) Confocal microscopy images of untransduced control neurons, alpha-Syn-transduced neurons treated with solvent, and alpha-Syn-transduced neurons treated with vinpocetine six days after transduction, stained with DAPI (blue), an alpha-Syn-antibody (green), and a beta-III-tubulin-antibody to demonstrate the axonal network (red). Scale bar: 20 um. ( c ) Quantification of the activated caspases signal after CellEvent TM staining in untransduced control neurons (left bar), alpha-Syn-transduced neurons treated with solvent (black bar), and alpha-Syn-transduced neurons treated with vinpocetine (red bar) showing activation of caspases 3 and 7 in alpha-Syn overexpressing neurons, which was reduced by treatment with 20 uM vinpocetine. ( d ) Representative images of the CellEvent TM staining. Scale bar 50 um. ( e ) Representative Western blot with an alpha-Syn antibody (C20, Santa Cruz) in lysates of control neurons and alpha-Syn-transduced neurons treated with solvent or with vinpocetine at different concentrations. Th
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
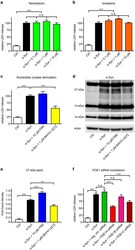
- Experimental details
- Figure 4 Mechanisms of action relevant for vinpocetine's efficacy. ( a - d ) LDH release was used to measure degeneration in untransduced control neurons (Ctrl, white bars), alpha-Syn-transduced neurons treated with solvent (black bars), and alpha-Syn-transduced neurons with different interventions (coloured bars). The sodium channel blocker tetrodotoxin ( a , green bars) and the L-type calcium channel blocker isradipine ( b , orange bars) in different concentrations did not protect against alpha-Syn-incuded toxicity. The adenylate cyclase stimulator forskolin ( c , FRK, blue bar) did also not protect, but the guanylate cyclase stimulator BAY41-2272 did ( c , yellow bar). ( d ) Western blot with an alpha-Syn antibody (C20, Santa Cruz) of untransduced control neurons (Ctrl), alpha-Syn-transduced neurons treated with solvent (second lane), and alpha-Syn-transduced neurons with forskolin (FRK) or BAY41-2272 showed that BAY41-2272 treatment also led to a reduction of a 37 kDa alpha-Syn band, while forskolin led to an increase of this band. ( e ) Quantification of the 37 kDa band. ( f ) A negative control siRNA (green bar) and siRNA against PDE1B (pink bar) did not protect, but siRNA against PDE1A (bright red bar) was strongly protective, and siRNA against PDE1C (dark red bar) was moderately protective. * P < 0.05, ** P < 0.01, *** P < 0.001, n.s. not significant, one-way ANOVA with Tukey's HSD post-hoc test. N-values, F-values and degrees of freedom (DF): ( a ) N = 7, F
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
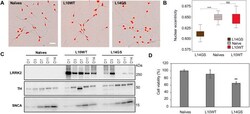
- Experimental details
- Fig. 3. LUHMES cell clones differentiate into neurons and express dopaminergic markers. (A) Morphological analysis of naive and LRRK2-expressing LUHMES clones (L10WT and L14GS) was performed in live cells using the Incucyte S3 analysis system. Several parameters were analyzed with the Incucyte Neurotracker software using brightfield images. Nuclei were stained with the NucLight reagent (red). The displayed experiment is representative of two independent experiments. Scale bar: 25 um. (B) Differences in nuclear shape (eccentricity) were statistically significant between the G2019S clone L14GS and the naive LUHMES cells. WT LUHMES clone (L10WT) behaved similarly to the naive cells. Error bars show mean+-s.d. One-way ANOVA with Tukey's post-hoc test was performed. Differences with P =10 from two independent experiments. *** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
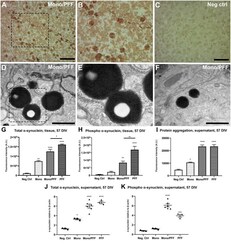
- Experimental details
- Exogenous alpha-synuclein is taken up by organotypic slices, causing accumulation, aggregation and phosphorylation. (A) Representative immunohistochemical staining of organotypic slice cultures treated with monomeric/PFF alpha-synuclein showing the presence of pS129 (phosphorylated alpha-synuclein) positive neurons and neurites following 57 DIV. Scale bar = 50 mum. (B) 2 x Magnified image of marked area in (A) . (C) Untreated negative control with no identified pS129 labeled structures organotypic slice cultures following 57 DIV. Scale bar = 50 mum. (D) Representative TEM of organotypic slice cultures treated with monomeric/PFF alpha-synuclein and following 57 DIV. Note the LB-like electron dense inclusion bodies in organotypic slice cultures. Scale bar = 1 mum. (E) Magnification from marked area in (D) , note the amorphous granular composition with autophagosomes double membrane. (F) Representative TEM of Lewy-bodies like electron-dense inclusion bodies in organotypic slice cultures treated with monomeric/PFF alpha-synuclein, following 57 DIV. Scale bar = 1 mum. (G) Semi-quantitative analysis of alpha-synuclein immunofluorescence in organotypic slice cultures following 57 DIV ( n >= 8). (H) Semi-quantitative analysis of phosphorylated alpha-synuclein immunofluorescence organotypic slice cultures following 57 DIV ( n >= 7). (I) Levels of total aggregated protein were determined within the supernatant of organotypic slice cultures following 57 DIV by fluorometric Proteostat(r)
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry