Antibody data
- Antibody Data
- Antigen structure
- References [10]
- Comments [0]
- Validations
- Immunocytochemistry [12]
- Immunoprecipitation [1]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-117 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PAX7 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Purifed from natural sources
- Description
- Western blot analysis of PA1-117 detects a prominent ~54 kDa protein in human HeLa, MCF7, rhabdomyosarcoma and skeletal muscle satellite cells and a weaker ~57 kDa protein in HeLa, rhabdomyosarcoma and skeletal muscle satellite cells. The ~57 kDa band seems to be enriched in immunoprecipitations from HeLa lysate, suggesting a bias for the larger isoform in ths application. Weak unknown bands of lower molecular weight and at ~110-130 kDa are also detected. Specificity of the antibody was also confirmed in Hela cells overexpressing full length PAX7.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C
Submitted references Uhrf1 governs the proliferation and differentiation of muscle satellite cells.
G-quadruplex DNA structures in human stem cells and differentiation.
Dysregulation of Muscle-Specific MicroRNAs as Common Pathogenic Feature Associated with Muscle Atrophy in ALS, SMA and SBMA: Evidence from Animal Models and Human Patients.
Meflin defines mesenchymal stem cells and/or their early progenitors with multilineage differentiation capacity.
Interference with SRF expression in skeletal muscles reduces peripheral nerve regeneration in mice.
Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing.
Age-dependent increase in angiopoietin-like protein 2 accelerates skeletal muscle loss in mice.
Histone H3.3 sub-variant H3mm7 is required for normal skeletal muscle regeneration.
Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice.
Enobosarm (GTx-024) Modulates Adult Skeletal Muscle Mass Independently of the Androgen Receptor in the Satellite Cell Lineage.
Sakai H, Sawada Y, Tokunaga N, Tanaka K, Nakagawa S, Sakakibara I, Ono Y, Fukada SI, Ohkawa Y, Kikugawa T, Saika T, Imai Y
iScience 2022 Mar 18;25(3):103928
iScience 2022 Mar 18;25(3):103928
G-quadruplex DNA structures in human stem cells and differentiation.
Zyner KG, Simeone A, Flynn SM, Doyle C, Marsico G, Adhikari S, Portella G, Tannahill D, Balasubramanian S
Nature communications 2022 Jan 10;13(1):142
Nature communications 2022 Jan 10;13(1):142
Dysregulation of Muscle-Specific MicroRNAs as Common Pathogenic Feature Associated with Muscle Atrophy in ALS, SMA and SBMA: Evidence from Animal Models and Human Patients.
Malacarne C, Galbiati M, Giagnorio E, Cavalcante P, Salerno F, Andreetta F, Cagnoli C, Taiana M, Nizzardo M, Corti S, Pensato V, Venerando A, Gellera C, Fenu S, Pareyson D, Masson R, Maggi L, Dalla Bella E, Lauria G, Mantegazza R, Bernasconi P, Poletti A, Bonanno S, Marcuzzo S
International journal of molecular sciences 2021 May 26;22(11)
International journal of molecular sciences 2021 May 26;22(11)
Meflin defines mesenchymal stem cells and/or their early progenitors with multilineage differentiation capacity.
Hara A, Kato K, Ishihara T, Kobayashi H, Asai N, Mii S, Shiraki Y, Miyai Y, Ando R, Mizutani Y, Iida T, Takefuji M, Murohara T, Takahashi M, Enomoto A
Genes to cells : devoted to molecular & cellular mechanisms 2021 Jul;26(7):495-512
Genes to cells : devoted to molecular & cellular mechanisms 2021 Jul;26(7):495-512
Interference with SRF expression in skeletal muscles reduces peripheral nerve regeneration in mice.
Wanner R, Knöll B
Scientific reports 2020 Mar 24;10(1):5281
Scientific reports 2020 Mar 24;10(1):5281
Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing.
Akiyama T, Sato S, Chikazawa-Nohtomi N, Soma A, Kimura H, Wakabayashi S, Ko SBH, Ko MSH
Scientific reports 2018 Jan 19;8(1):1189
Scientific reports 2018 Jan 19;8(1):1189
Age-dependent increase in angiopoietin-like protein 2 accelerates skeletal muscle loss in mice.
Zhao J, Tian Z, Kadomatsu T, Xie P, Miyata K, Sugizaki T, Endo M, Zhu S, Fan H, Horiguchi H, Morinaga J, Terada K, Yoshizawa T, Yamagata K, Oike Y
The Journal of biological chemistry 2018 Feb 2;293(5):1596-1609
The Journal of biological chemistry 2018 Feb 2;293(5):1596-1609
Histone H3.3 sub-variant H3mm7 is required for normal skeletal muscle regeneration.
Harada A, Maehara K, Ono Y, Taguchi H, Yoshioka K, Kitajima Y, Xie Y, Sato Y, Iwasaki T, Nogami J, Okada S, Komatsu T, Semba Y, Takemoto T, Kimura H, Kurumizaka H, Ohkawa Y
Nature communications 2018 Apr 11;9(1):1400
Nature communications 2018 Apr 11;9(1):1400
Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice.
Rozo M, Li L, Fan CM
Nature medicine 2016 Aug;22(8):889-96
Nature medicine 2016 Aug;22(8):889-96
Enobosarm (GTx-024) Modulates Adult Skeletal Muscle Mass Independently of the Androgen Receptor in the Satellite Cell Lineage.
Dubois V, Simitsidellis I, Laurent MR, Jardi F, Saunders PT, Vanderschueren D, Claessens F
Endocrinology 2015 Dec;156(12):4522-33
Endocrinology 2015 Dec;156(12):4522-33
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
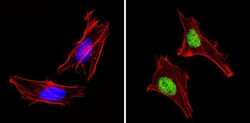
- Experimental details
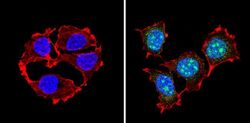
- Immunofluorescent analysis of PAX7 (green) showing staining in the in the nucleus of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
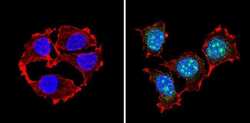
- Experimental details
- Immunofluorescent analysis of PAX7 (green) showing staining in the in the nucleus of MCF-7 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
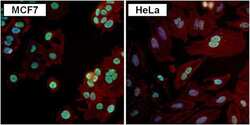
- Experimental details
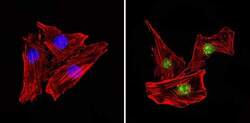
- Immunofluorescent analysis of PAX7 (green) in MCF7 and HeLa cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature. Cells were blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBS, and incubated with a DyLight 488-conjugated goat anti-rabbit IgG secondary antibody (Product # 35552) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with DyLight-554 Phalloidin (Product # 21834) and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ToxInsight Instrument at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
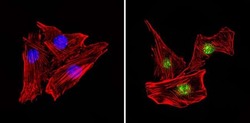
- Experimental details
- Immunofluorescent analysis of PAX7 (green) showing staining in the in the nucleus of C2C12 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
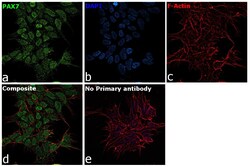
- Experimental details
- Immunofluorescence analysis of PAX7 was performed using 70% confluent log phase SK-N-MC cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with PAX7 Polyclonal Antibody (Product # PA1-117) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
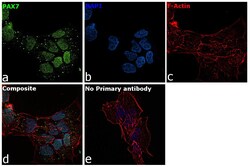
- Experimental details
- Immunofluorescence analysis of PAX7 was performed using 70% confluent log phase A-673 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with PAX7 Polyclonal Antibody (Product # PA1-117) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained withRhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of PAX7 (green) showing staining in the in the nucleus of MCF-7 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of PAX7 (green) showing staining in the in the nucleus of C2C12 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
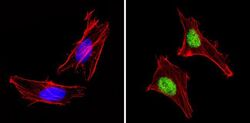
- Immunofluorescent analysis of PAX7 (green) showing staining in the in the nucleus of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
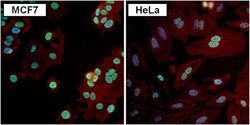
- Immunofluorescent analysis of PAX7 (green) in MCF7 and HeLa cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature. Cells were blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with a PAX7 polyclonal antibody (Product # PA1-117) at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBS, and incubated with a DyLight 488-conjugated goat anti-rabbit IgG secondary antibody (Product # 35552) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with DyLight-554 Phalloidin (Product # 21834) and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ToxInsight Instrument at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
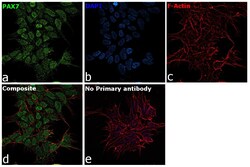
- Experimental details
- Immunofluorescence analysis of PAX7 was performed using 70% confluent log phase SK-N-MC cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with PAX7 Polyclonal Antibody (Product # PA1-117) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
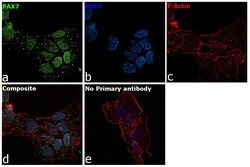
- Experimental details
- Immunofluorescence analysis of PAX7 was performed using 70% confluent log phase A-673 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 1 hour at room temperature. The cells were labeled with PAX7 Polyclonal Antibody (Product # PA1-117) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32790), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained withRhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
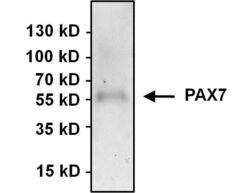
- Experimental details
- Immunoprecipitation of PAX7 was performed using HeLa whole cell lysate. Antigen-antibody complexes were formed by incubating 500 µg of lysate with 3 µg of a PAX7 polyclonal antibody (Product # PA1-117) overnight on a rocking platform at 4°C. The immune complexes were captured on 50 µL Protein A/G Agarose (Product # 20421), washed extensively, and eluted with 5X Lane Marker Reducing Sample Buffer (Product # 39000). Samples was resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBS-0.1%Tween-20 for 1 hour. The membrane was probed with a PAX7 polyclonal antibody (Product # PA1-117) at a dilution of 1:1000 overnight rotating at 4°C. Membranes were washed in TBST, and probed with Clean-Blot IP Detection Reagent (Product # 21230) at a dilution of 1:1000 for at least one hour. Chemiluminescent detection was performed using SuperSignal West Pico (Product # 34080).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
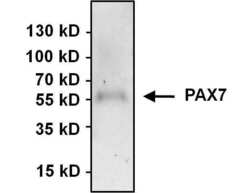
- Experimental details
- Immunoprecipitation of PAX7 was performed using HeLa whole cell lysate. Antigen-antibody complexes were formed by incubating 500 µg of lysate with 3 µg of a PAX7 polyclonal antibody (Product # PA1-117) overnight on a rocking platform at 4øC. The immune complexes were captured on 50 µL Protein A/G Agarose (Product # 20421), washed extensively, and eluted with 5X Lane Marker Reducing Sample Buffer (Product # 39000). Samples was resolved on a 4-20% Tris-HCl polyacrylamide gel, transferred to a PVDF membrane, and blocked with 5% BSA/TBS-0.1%Tween-20 for 1 hour. The membrane was probed with a PAX7 polyclonal antibody (Product # PA1-117) at a dilution of 1:1000 overnight rotating at 4øC. Membranes were washed in TBST, and probed with Clean-Blot IP Detection Reagent (Product # 21230) at a dilution of 1:1000 for at least one hour. Chemiluminescent detection was performed using SuperSignal West Pico (Product # 34080).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
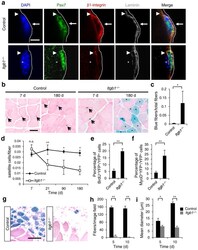
- Figure 1 Young Itgb1 -/- SCs are defective in maintaining quiescence and sustaining regeneration. ( a ) Control and Itgb1 -/- SCs on myofibers (assay scheme in Supplementary Fig. 1a ) stained for Pax7, beta1-integrin, and laminin (dotted line); arrows, basal beta1-integrin in SC; arrowheads, muscle beta1-integrin; asterisks, myonucleus; Scale bar, 5 mum. ( b - d ) Long-term tracing of beta-gal lineage-marked ( R26R LacZ reporter) control and mutant SCs after tmx regimen: ( b ) X-gal reacted (blue) and nuclear fast red stained muscle sections at 7 and 180 d; arrows, SCs; asterisks, X-gal + myofibers. ( c ) Quantified blue fibers per field using data in ( b ). ( d ) SC to fiber ratios at 7, 21, 90, and 180 d; n = 3 animals per group, ten sections scored per animal. ( e ) Percentages of BrdU + YFP + /total YFP + cells of control and Itgb1 -/- muscles after 1 month of BrdU administration; n = 3 animals per group, ten sections scored per animal. ( f ) Percentages of MyoD + YFP + /YFP + cells from the same animals in e . ( g ) Representative images ( n > 15 per condition) of X-gal reacted control and Itgb1 -/- muscle sections at 5 d post CTX injury (scheme in Supplementary Fig. 3a ); Scale bar, 25 mum. ( h , i ) Average fiber number per field (0.228 mum 2 ) ( h ) and mean fiber diameter ( i ) at 5 d and 10 d post injury; n = 3 animals per group, 20 sections scored per animal. All numerical data are presented as mean +- s.d.; Student's t -test: * P < 0.05, ** P < 0.01, and n.s., not
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
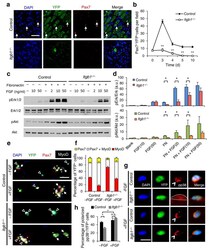
- Figure 3 Itgb1 - / - SCs show a compromised response to FGF-2 that can be partially restored by exogenous FGF-2. ( a ) Control and Itgb1 -/- sections 10 d after injury stained for Pax7 and YFP; arrows, Pax7 + SCs; Scale bar, 25 mum. ( b ) Average number of Pax7 + YFP + cells per field (0.228 mum 2 ) during regeneration; n = 3 animals per time point, ten sections per animal; data are expressed as mean +- s.d.; Student's t -test: * P < 0.05; ** P < 0.01. ( c ) Western blots for pErk1 and pErk2, Erk1 and Erk2, pAkt, and Akt of control and Itgb1 -/- cells. Fibronectin addition (+) and FGF-2 concentrations (FGF(ng/ml)) are indicated. ( d ) Fold induction from data in c , normalized to control cells without fibronectin and FGF-2 (set at 1 arbitrary unit (a.u.)); n = 4 parallel sets of myoblasts. Paired comparisons with significant differences are indicated; data are expressed as mean +- s.e.m.; two-way ANOVA: * P < 0.05. ( e ) Control and Itgb1 -/- SCs cultured for 96 h with or without 10 ng/ml FGF-2 were stained for YFP, Pax7, MyoD, and DAPI; arrows, Pax7 + cells, asterisks, Pax7 + MyoD + cells, and triangles, MyoD + cells; scale bar = 25 mum. ( f ) Percentage distribution of various cell populations from data in ( e ); n = 3 animals; >= 20 myofibers per condition; two-way ANOVA: P < 0.01 for Pax7 + MyoD + and MyoD + , -FGF vs. +FGF (control and Itgb1 -/- ), control vs. Itgb1 -/- (-FGF and +FGF); P < 0.05 for Pax7 + , control vs. Itgb1 -/- (-FGF). ( g ) Representative images ( n =
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
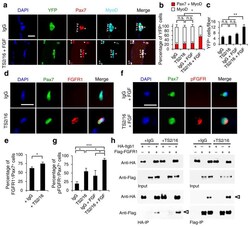
- Figure 5 Activating beta1-integrin in aged SCs enhances FGF signaling to promote SC expansion. ( a - c ) Myofiber-associated YFP + aged SCs were cultured with IgG or TS2/16 (at 10 mug/ml) and with or without FGF-2 (FGF, 10 ng/ml) for 72 h, and stained for Pax7 and MyoD. ( a ) Images for IgG alone and TS2/16 + FGF-2 treated cultures; asterisk, Pax7 + MyoD + cells; open arrowhead, MyoD + cells; Scale bar, 25 mum. ( b ) Percentages of Pax7 + MyoD + versus MyoD + cells in all four groups; mean +- s.d.; n = 3 experiments, >= 30 myofibers each; two-way ANOVA: * P < 0.05 for TS2/16 + FGF-2 versus others, and not significant (n.s.) between the other groups. ( c ) Average number of YFP + cells per myofiber of the same samples in a ; mean +- s.d.; n = 3 animals, >= 15 myofibers each two-way ANOVA: ** P < 0.01 for TS2/16 + FGF-2 versus others, and n.s. between the other groups. ( d , e ) Myofiber-associated aged SCs cultured for 24 h with IgG or TS2/16 and stained for Pax7 and FGFR1. ( d ) Representative images ( n = 20); Scale bar, 10 mum. ( e ) Percentage of FGFR1 + cells within the Pax7 + population; mean +- s.d., n = 3 experiments, >= 20 myofibers per condition; Student's t -test: * P < 0.05. ( f , g ) Aged SCs cultured with IgG or TS2/16, and with or without FGF-2, were stained for phospho-FGFR (pFGFR) and Pax7. ( f ) Representative images of IgG + FGF-2 and TS2/16 + FGF-2 ( n = 20 images of each group); Scale bar, 10 mum. ( g ) Percentage of pFGFR + cells within the Pax7 + SC popu
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
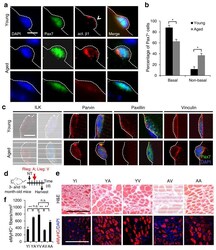
- Figure 4 Activating beta1-integrin in aged SCs can rescue aged-associated SC defects. ( a , b ) Myofiber-associated young and aged SCs stained for Pax7, activated beta1-integrin (act. beta1), and DAPI 1 h after isolation; basal surface, dashed line; act. beta1 patterns scored as basal (open arrowhead) or unevenly or non-detectable (non-basal) in b ; Scale bar, 10 mum. All images were taken with same exposure. ( b ) Percentages of Pax7 + SCs scored by act. beta1 patterns from a ; n = 3 experiments, >= 20 myofibers; numerical data are expressed as mean +- s.d.; Student's t -test: * P < 0.05. ( c ) Young and aged Pax7 + SCs stained for ILK, Parvin, Paxillin, Vinculin, and DAPI 1 h after isolation; dashed lines, basal surface; scale bar = 5 mum. All images were taken with same exposure. ( d ) Schematic for beta1-integrin activation in young (3 month) or aged (18 month) muscles after needle track injury. 2 d post injury, IgG vehicle (10 mug/ml; V), TS2/16 activating antibody (10 mug/ml; A), or RGD peptide inhibitor (10 mug/m; I) were injected into the injury site. Muscles were harvested 3 d later. ( e ) Muscle sections were stained for H&E or eMyHC; Scale bar, 150 mum. ( f ) Average number of eMyHC + fibers in injured areas of each group; data represent mean +- s.d.; n = 3; ten sections per animal; two-way ANOVA: ** P < 0.01 and n.s., not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
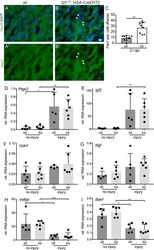
- Figure 6 Enhanced SC numbers but no changes in growth factor mRNA levels after FN injury in SRF deficient animals. ( A-C ) Masseter muscles at 21 dpi were stained for the SC marker Pax7 (green), and DAPI (blue). Pax7 abundance was elevated in SRF depleted muscles ( B ) compared to wt myofibers. (A'',B'') show the Pax7 channel of wt ( A ) and Srf mutant ( B ) only. Quantification of Pax7 positive cells/area ( C ). ( D-I ) cDNA of uninjured and injured masseter muscles of wt and Srf loxp/loxp : HSA-CreERT2 mutant animals (ko) was subjected to qPCR analysis for genes indicated at 7 dpi. Ptgs2 ( D ), Igf2 ( E ) were strongly and Gdnf ( F ) and Ngf ( G ) were mildly upregulated by injury with no differences between genotypes. Vegfb ( H ) and Bdnf ( I ) were downregulated by injury in a comparable manner between wt and ko animals. Each black circle in a bar reflects one animal analyzed. Data were provided as mean +- SD. *P < 0.05, **P < 0.001, ***P < 0.001. Scale-bar ( A , B ) = 30 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
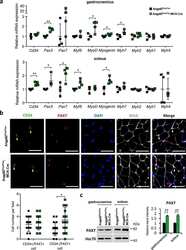
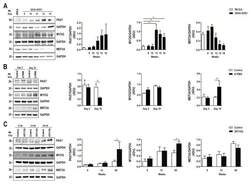
- Figure 3 Altered expression of PAX7, MYOG, and MEF2A proteins in G93A-SOD1, Delta7SMA, and AR113Q mouse muscle as disease progresses. Representative western blot analysis of PAX7, MYOG, and MEF2A proteins in gastrocnemius muscle tissue of ( A ) G93A-SOD1 (black bars), ( B ) Delta7SMA (black bars), and ( C ) AR113Q (black bars) mice and control mice (white bars) ( n = 3 mice per group) with relative densitometric analysis. Density values are reported as mean +- SEM, corrected for background and normalized to GAPDH control. * p < 0.05, Mann-Whitney test.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry