Antibody data
- Antibody Data
- Antigen structure
- References [27]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [11]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 11-0116-41 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD11c Monoclonal Antibody (3.9), FITC, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The 3.9 monoclonal antibody reacts with human CD11c, the 150 kDa integrin alpha X chain. CD11c non-covalently associates with beta2 integrin to form the CD11c/CD18 heterodimer. This complex is expressed on monocytes, granulocytes, macrophages, NK, dendritic cells and subset of T and B lymphocytes. CD11c/CD18 binds to CD54, iC3b and fibrinogen and plays a role in leukocyte adhesive interactions. Applications Reported: The 3.9 antibody has been reported for use in flow cytometric analysis. Applications Tested: This 3.9 antibody has been pre-titrated and tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at 5 µL (1 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Excitation: 488 nm; Emission: 520 nm; Laser: Blue Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Conjugate
- Green dye
- Isotype
- IgG
- Antibody clone number
- 3.9
- Vial size
- 25 Tests
- Concentration
- 5 µL/Test
- Storage
- 4° C, store in dark, DO NOT FREEZE!
Submitted references Aluminum hydroxide adjuvant diverts the uptake and trafficking of genetically detoxified pertussis toxin to lysosomes in macrophages.
Therapeutic inhibition of the SRC-kinase HCK facilitates T cell tumor infiltration and improves response to immunotherapy.
Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus.
Upregulation of CD3ζ and L-selectin in antigen-specific cytotoxic T lymphocytes by eliminating myeloid-derived suppressor cells with doxorubicin to improve killing efficacy of neuroblastoma cells in vitro.
Increased TNF-α Initiates Cytoplasmic Vacuolization in Whole Blood Coculture with Dengue Virus.
SUMOylation disassembles the tetrameric pyruvate kinase M2 to block myeloid differentiation of leukemia cells.
Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells.
Biological Therapy in Inflammatory Bowel Disease Patients Partly Restores Intestinal Innate Lymphoid Cell Subtype Equilibrium.
The long non-coding RNA Cancer Susceptibility 15 (CASC15) is induced by isocitrate dehydrogenase (IDH) mutations and maintains an immature phenotype in adult acute myeloid leukemia.
Human and Mouse Transcriptome Profiling Identifies Cross-Species Homology in Pulmonary and Lymph Node Mononuclear Phagocytes.
FAT10 localises in dendritic cell aggresome-like induced structures and contributes to their disassembly.
Comprehensive Cell Surface Antigen Analysis Identifies Transferrin Receptor Protein-1 (CD71) as a Negative Selection Marker for Human Neuronal Cells.
CD62L Is a Functional and Phenotypic Marker for Circulating Innate Lymphoid Cell Precursors.
AG490 reverses phenotypic alteration of dendritic cells by bladder cancer cells.
FcαRI co-stimulation converts human intestinal CD103(+) dendritic cells into pro-inflammatory cells through glycolytic reprogramming.
Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2.
Rv2299c, a novel dendritic cell-activating antigen of Mycobacterium tuberculosis, fused-ESAT-6 subunit vaccine confers improved and durable protection against the hypervirulent strain HN878 in mice.
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis.
Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia.
Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti-helminthic treatment.
Towards programming immune tolerance through geometric manipulation of phosphatidylserine.
CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22.
Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling.
Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes.
Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPα.
Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans.
Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy.
Jaldin-Fincati J, Moussaoui S, Gimenez MC, Ho CY, Lancaster CE, Botelho R, Ausar F, Brookes R, Terebiznik M
Molecular microbiology 2022 May;117(5):1173-1195
Molecular microbiology 2022 May;117(5):1173-1195
Therapeutic inhibition of the SRC-kinase HCK facilitates T cell tumor infiltration and improves response to immunotherapy.
Poh AR, Love CG, Chisanga D, Steer JH, Baloyan D, Chopin M, Nutt S, Rautela J, Huntington ND, Etemadi N, O'Brien M, O'Keefe R, Ellies LG, Macri C, Mintern JD, Whitehead L, Gangadhara G, Boon L, Chand AL, Lowell CA, Shi W, Pixley FJ, Ernst M
Science advances 2022 Jun 24;8(25):eabl7882
Science advances 2022 Jun 24;8(25):eabl7882
Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus.
Patrick DM, de la Visitación N, Krishnan J, Chen W, Ormseth MJ, Stein CM, Davies SS, Amarnath V, Crofford LJ, Williams JM, Zhao S, Smart CD, Dikalov S, Dikalova A, Xiao L, Van Beusecum JP, Ao M, Fogo AB, Kirabo A, Harrison DG
JCI insight 2022 Jul 8;7(13)
JCI insight 2022 Jul 8;7(13)
Upregulation of CD3ζ and L-selectin in antigen-specific cytotoxic T lymphocytes by eliminating myeloid-derived suppressor cells with doxorubicin to improve killing efficacy of neuroblastoma cells in vitro.
Xu W, Li S, Li M, Zhou H, Yang X
Journal of clinical laboratory analysis 2022 Jan;36(1):e24158
Journal of clinical laboratory analysis 2022 Jan;36(1):e24158
Increased TNF-α Initiates Cytoplasmic Vacuolization in Whole Blood Coculture with Dengue Virus.
Satria RD, Huang TW, Jhan MK, Shen TJ, Tseng PC, Wang YT, Yang ZY, Hsing CH, Lin CF
Journal of immunology research 2021;2021:6654617
Journal of immunology research 2021;2021:6654617
SUMOylation disassembles the tetrameric pyruvate kinase M2 to block myeloid differentiation of leukemia cells.
Xia L, Jiang Y, Zhang XH, Wang XR, Wei R, Qin K, Lu Y
Cell death & disease 2021 Jan 20;12(1):101
Cell death & disease 2021 Jan 20;12(1):101
Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells.
Li L, Liao Z, Ye M, Jiang J
Reproductive biology and endocrinology : RB&E 2021 Aug 24;19(1):128
Reproductive biology and endocrinology : RB&E 2021 Aug 24;19(1):128
Biological Therapy in Inflammatory Bowel Disease Patients Partly Restores Intestinal Innate Lymphoid Cell Subtype Equilibrium.
Creyns B, Jacobs I, Verstockt B, Cremer J, Ballet V, Vandecasteele R, Vanuytsel T, Ferrante M, Vermeire S, Van Assche G, Ceuppens JL, Breynaert C
Frontiers in immunology 2020;11:1847
Frontiers in immunology 2020;11:1847
The long non-coding RNA Cancer Susceptibility 15 (CASC15) is induced by isocitrate dehydrogenase (IDH) mutations and maintains an immature phenotype in adult acute myeloid leukemia.
Grasedieck S, Ruess C, Krowiorz K, Lux S, Pochert N, Schwarzer A, Klusmann JH, Jongen-Lavrencic M, Herold T, Bullinger L, Pollack JR, Rouhi A, Kuchenbauer F
Haematologica 2020 Sep 1;105(9):e448-453
Haematologica 2020 Sep 1;105(9):e448-453
Human and Mouse Transcriptome Profiling Identifies Cross-Species Homology in Pulmonary and Lymph Node Mononuclear Phagocytes.
Leach SM, Gibbings SL, Tewari AD, Atif SM, Vestal B, Danhorn T, Janssen WJ, Wager TD, Jakubzick CV
Cell reports 2020 Nov 3;33(5):108337
Cell reports 2020 Nov 3;33(5):108337
FAT10 localises in dendritic cell aggresome-like induced structures and contributes to their disassembly.
Schregle R, Mueller S, Legler DF, Rossy J, Krueger WA, Groettrup M
Journal of cell science 2020 Jul 16;133(14)
Journal of cell science 2020 Jul 16;133(14)
Comprehensive Cell Surface Antigen Analysis Identifies Transferrin Receptor Protein-1 (CD71) as a Negative Selection Marker for Human Neuronal Cells.
Menon V, Thomas R, Elgueta C, Horl M, Osborn T, Hallett PJ, Bartos M, Isacson O, Pruszak J
Stem cells (Dayton, Ohio) 2019 Oct;37(10):1293-1306
Stem cells (Dayton, Ohio) 2019 Oct;37(10):1293-1306
CD62L Is a Functional and Phenotypic Marker for Circulating Innate Lymphoid Cell Precursors.
Bar-Ephraim YE, Koning JJ, Burniol Ruiz E, Konijn T, Mourits VP, Lakeman KA, Boon L, Bögels M, van Maanen JP, Den Haan JMM, van Egmond M, Bouma G, Reijmers RM, Mebius RE
Journal of immunology (Baltimore, Md. : 1950) 2019 Jan 1;202(1):171-182
Journal of immunology (Baltimore, Md. : 1950) 2019 Jan 1;202(1):171-182
AG490 reverses phenotypic alteration of dendritic cells by bladder cancer cells.
Xiu W, Ma J, Lei T, Zhang M
Oncology letters 2018 Sep;16(3):2851-2856
Oncology letters 2018 Sep;16(3):2851-2856
FcαRI co-stimulation converts human intestinal CD103(+) dendritic cells into pro-inflammatory cells through glycolytic reprogramming.
Hansen IS, Krabbendam L, Bernink JH, Loayza-Puch F, Hoepel W, van Burgsteden JA, Kuijper EC, Buskens CJ, Bemelman WA, Zaat SAJ, Agami R, Vidarsson G, van den Brink GR, de Jong EC, Wildenberg ME, Baeten DLP, Everts B, den Dunnen J
Nature communications 2018 Feb 28;9(1):863
Nature communications 2018 Feb 28;9(1):863
Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2.
Annibaldi A, Wicky John S, Vanden Berghe T, Swatek KN, Ruan J, Liccardi G, Bianchi K, Elliott PR, Choi SM, Van Coillie S, Bertin J, Wu H, Komander D, Vandenabeele P, Silke J, Meier P
Molecular cell 2018 Feb 15;69(4):566-580.e5
Molecular cell 2018 Feb 15;69(4):566-580.e5
Rv2299c, a novel dendritic cell-activating antigen of Mycobacterium tuberculosis, fused-ESAT-6 subunit vaccine confers improved and durable protection against the hypervirulent strain HN878 in mice.
Choi HG, Choi S, Back YW, Paik S, Park HS, Kim WS, Kim H, Cha SB, Choi CH, Shin SJ, Kim HJ
Oncotarget 2017 Mar 21;8(12):19947-19967
Oncotarget 2017 Mar 21;8(12):19947-19967
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis.
Lim S, Kim WJ, Kim YH, Lee S, Koo JH, Lee JA, Yoon H, Kim DH, Park HJ, Kim HM, Lee HG, Yun Kim J, Lee JU, Hun Shin J, Kyun Kim L, Doh J, Kim H, Lee SK, Bothwell ALM, Suh M, Choi JM
Nature communications 2015 Sep 15;6:8244
Nature communications 2015 Sep 15;6:8244
Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia.
Khalife J, Radomska HS, Santhanam R, Huang X, Neviani P, Saultz J, Wang H, Wu YZ, Alachkar H, Anghelina M, Dorrance A, Curfman J, Bloomfield CD, Medeiros BC, Perrotti D, Lee LJ, Lee RJ, Caligiuri MA, Pichiorri F, Croce CM, Garzon R, Guzman ML, Mendler JH, Marcucci G
Leukemia 2015 Oct;29(10):1981-92
Leukemia 2015 Oct;29(10):1981-92
Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti-helminthic treatment.
Nausch N, Appleby LJ, Sparks AM, Midzi N, Mduluza T, Mutapi F
PLoS neglected tropical diseases 2015 Mar;9(3):e0003627
PLoS neglected tropical diseases 2015 Mar;9(3):e0003627
Towards programming immune tolerance through geometric manipulation of phosphatidylserine.
Roberts RA, Eitas TK, Byrne JD, Johnson BM, Short PJ, McKinnon KP, Reisdorf S, Luft JC, DeSimone JM, Ting JP
Biomaterials 2015 Dec;72:1-10
Biomaterials 2015 Dec;72:1-10
CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22.
Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR
The Journal of experimental medicine 2014 Jul 28;211(8):1571-83
The Journal of experimental medicine 2014 Jul 28;211(8):1571-83
Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling.
Fernandez I, Ooi TP, Roy K
Stem cells (Dayton, Ohio) 2014 Jan;32(1):93-104
Stem cells (Dayton, Ohio) 2014 Jan;32(1):93-104
Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes.
Sekine C, Koyanagi A, Koyama N, Hozumi K, Chiba S, Yagita H
Arthritis research & therapy 2012 Mar 5;14(2):R45
Arthritis research & therapy 2012 Mar 5;14(2):R45
Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPα.
Radomska HS, Alberich-Jordà M, Will B, Gonzalez D, Delwel R, Tenen DG
The Journal of clinical investigation 2012 Aug;122(8):2955-66
The Journal of clinical investigation 2012 Aug;122(8):2955-66
Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans.
Lay MK, Atmar RL, Guix S, Bharadwaj U, He H, Neill FH, Sastry KJ, Yao Q, Estes MK
Virology 2010 Oct 10;406(1):1-11
Virology 2010 Oct 10;406(1):1-11
Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy.
Kavousanaki M, Makrigiannakis A, Boumpas D, Verginis P
Arthritis and rheumatism 2010 Jan;62(1):53-63
Arthritis and rheumatism 2010 Jan;62(1):53-63
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
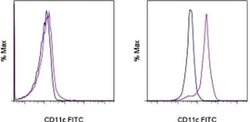
- Staining of normal human peripheral blood cells with Mouse IgG1 K Isotype Control FITC (Product # 11-4714-42) (blue histogram) or Anti-Human CD11c FITC (purple histogram). Cells in the lymphocyte gate (left) and monocyte gate (right) were used for analysis.
- Conjugate
- Green dye
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Immune profiling in DENV-treated whole blood cells 24 h postincubation. Following DENV (MOI = 1) coculture in 100 mu l of WB ex vivo for 24 h, (a) representative flow cytometric analysis and gating of various cells obtained from five cases, performed by staining for specific cell surface markers (CD4, CD8, CD11c, CD14, CD16, CD19, CD25, CD56, CD62L, and HLA-DR), in the DENV-infected and mock groups showed (b) the changes in the expression of specific immune cell populations as noted. (c) The results are shown as a percentage of the mean +- SD obtained from five cases. * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the mock group. R: region; WBC: white blood cell; bri: bright.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
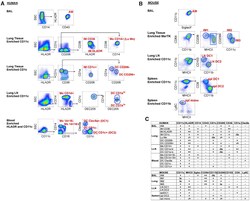
- Figure 1. Gating Strategy to Sort Human and Mouse Mononuclear Phagocytes for RNA Isolation (A and B) Fluorescence-activated cell sorting (FACS) gating strategy used to isolate human (A) or mouse (B) mononuclear phagocytes (MPs, labeled in red) from the indicated tissues. Before sorting, cell suspensions were magnetically enriched as indicated and gated to select Live; Single; CD45 + ; Lineage - cells as shown in Figures S1 and S2 . Arrows indicate where further analysis is performed on the specified subpopulation. The data presented are representative of 3-6 replicates per sort. (C) Table illustrating cell surface marker expression by MP subsets in human (top) and mouse (bottom). - or lo signifies no or little expression, + or ++ indicates expression or strong expression, -/+ indicates heterogeneous expression of marker proteins.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
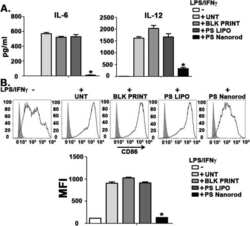
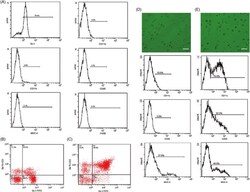
- FIGURE 1 Extraction, identification, and purification of myeloid-derived suppressor cell and cultivation of dendritic cell. (A) Cells were extracted from the bone marrow of BALB/c mice and stained by monoclonal antibodies. Under flow cytometry, the expressive rate of Gr-1 + MDSC, CD11b + MDSC, CD11c + MDSC, CD80 + MDSC, F4/80 + MDSC, and MHC-II + MDSC were 70.4%, 3.5%, 4.8%, 1.2%, 0.3%, 2.1% respectively. (B) The expressive rate of Gr-1 + CD11b + MDSC was 22.6%. (C) After MACS by CD11b magnetic bead, purification of Gr-1 + CD11b + MDSC reached 84.6%. (D) Most non-antigen-loaded dendritic cells grew adherently, with different sizes, star or spindle shape, and stretching tubers, but some of the cells seemed to have adopted a half-adherent state with rough surface. The expressive rates of CD11c, CD86, and MHC-II on DCs were 10.9%, 3.8%, and 27.9%, respectively, by flow cytometry. (E) On the 7th day, DCs were stimulated and activated by tumor antigens. DCs in the half-adherent state increased obviously with radial spikes and bigger shape. The expressive rates of CD11c, CD86, and MHC-II were 74.8%, 50.3%, and 49.8%, respectively, by flow cytometry. IgG FITC, a homotypic control antibody, was used to set the gate strategy. Scale bar = 100 mumol/liter. MDSC, myeloid-derived suppressor cell; MACS, magnetic-activated cell sorting; DC, dendritic cell; CD, cluster of differentiation
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
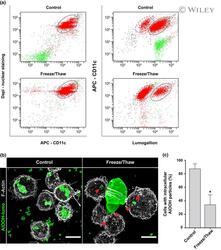
- 5 FIGURE Impact of particle size on aluminum adjuvant internalization. (a) U-937 cells were incubated with AlOOH-lumo (control) or AlOOH-lumo previously subjected to freeze/thaw cycles (freeze/thaw) to increase the size of the particles, as described in experimental procedures. The cells were incubated at 37degC for 4 h and subsequently processed for flow cytometry analysis. Differentiated macrophages were classified using anti-CD11c antibodies. Cell nuclei were labeled with DAPI. Cell populations encircled by a dotted line have not internalized AlOOH-Lumo and cells that have internalized AlOOH-Lumo are encircled by a solid line. (b) U-937 cells were incubated with control or freeze/thaw AlOOH-lumo preparations as described above and subsequently fixed and F-Actin stained with blue phalloidin (pseudocolored gray). Red arrowheads in the right panel point to small, internalized adjuvant particles. Spinning disk confocal images represent a merge of z-stacks. Images are representative of three independent trials. Fifty cells per trial per condition were analyzed. Scale bars, 5 mum. (c) U-937 cells were incubated with control or freeze/thaw AlOOH-lumo preparations and processed as described in (b). The percentage of cells with intracellular AlOOH particles were calculated for three independent trials and the mean represented in the bar graph. * p
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
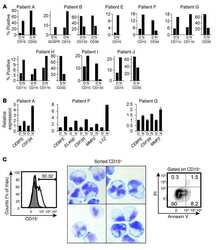
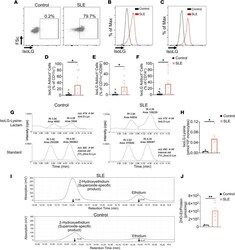
- IsoLG adducts are enriched in monocytes of patients with SLE. ( A ) Representative FACS plots displaying isoLG adduct containing CD11c + PBMCs from a representative control and patient with SLE. Representative histograms displaying the distribution of isoLG adducts in ( B ) CD11c + and ( C ) CD11c + CD86 + cells. Quantitation of IsoLG adduct-containing cells as a percentage of ( D ) CD11c + , ( E ) CD11c + CD86 + , and ( F ) CD14 + cells. For B - F data were analyzed using 1-tailed Student's t test or Mann-Whitney U test ( n = 10-11, *P < 0.05). ( G ) Stable isotope dilution multiple reaction monitoring for mass spectrometry analysis of isoLG-lysine-lactam adduct in DCs. Representative liquid chromatography/mass spectrometry chromatographs from a representative patient. The top row shows multiple reaction monitoring chromatographs for isoLG lysine lactam in samples, while the bottom row shows multiple reaction monitoring chromatograph for [13C615N2] internal standard for the same samples. cps, counts per second; Rt, retention time. ( H ) Quantitation of isoLG-lysine in monocytes from a subset of SLE patients and controls. ( I ) Monocytes from SLE patients and controls were sorted. Superoxide was detected using HPLC to monitor conversion of dihydroethidium to the superoxide oxidation adduct 2-hydroxyethidium (2-HO-ET) and ethidium. ( J ) Quantitation of 2-HO-ET from SLE patients and controls. For H and J , comparisons were made with a 1-tailed Student's t test ( n = 4-6, *P
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
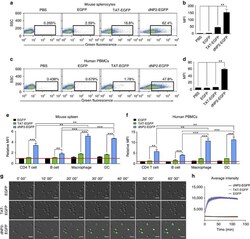
- Figure 2 Protein delivery efficiency of dNP2 in primary mouse and human immune cells. ( a , b ) Mouse primary splenocytes were isolated from 6-week-old female C57BL/6 mice and the cells were incubated with 5 muM EGFP, TAT- and dNP2-EGFP for 2 h. Intracellular fluorescence was analysed by flow cytometry and the data are represented as dot plots or mean fluorescence intensity (MFI) of the cells. ( c , d ) Human PBMCs were isolated from healthy donor blood and the cells were incubated with 5 muM EGFP, TAT-, dNP2-EGFP for 2 h. The data were analysed as described above. ( e ) Total splenocytes were incubated with 1 muM EGFP, TAT-, and dNP2-EGFP for 2 h. Cells were gated using markers specific for CD4 T cells (CD4 + ), B cells (CD19 + ), macrophages (CD11c lo CD11b hi F480 + ) and DCs (CD11c hi MHCII hi ). The EGFP signal in each cell population was then analysed by flow cytometric analysis. The relative MFI value was normalization to PBS treated cells. The red line indicates relative MFI of PBS-treated cells. ( f ) Total PBMCs were incubated with 1 muM EGFP, TAT-, and dNP2-EGFP for 2 h. Cells were gated with markers specific for CD4 T cells (CD4 + ), B cells (CD19 + ), macrophages (CD11b + ) and DCs (CD11c + ) and the data were then analysed as described above. ( g ) Time-lapse images of mouse CD4 T cells incubated with 1 muM EGFP, TAT- and dNP2-EGFP were acquired for 2 h (Scale bar, 15 mum) and ( h ) the average fluorescence intensities of 10 cells from each sample were calculate
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
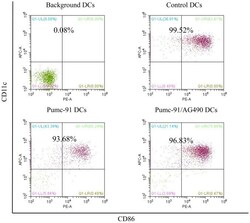
- Figure 2. Percentage of purified DC samples in all experiments. DCs were labeled with the Non-DC Depletion Cocktail for negative selection. Next, DCs were labeled with DC Enrichment Cocktail for positive selection. The expression of CD11c and CD86 was tested by flow cytometery. The percentages of purified DC are presented in the top of each panel. DCs, dendritic cells; CD, cluster of differentiation.
- Conjugate
- Green dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
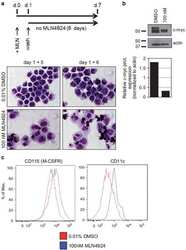
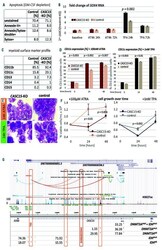
- Figure 1. CASC15 -KO promotes the differentiation of acute myeloid leukemia cells. (A) Apoptosis in CASC15 -KO and empty vector-transduced (control) OCI-AML5 cell lines after 24 h of depletion of granulocyte-macrophage colony-stimulating factor (annexin-FITC/Sytox blue flow cytometry). (B) Expression of SOX4 during in vitro differentiation of CASC15 -KO and control OCI-AML5 cell lines. All cells were treated with 0.1 mM all- trans retinoic acid (ATRA) and 1 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) over 72 h in three independent experiments. Total RNA was extracted before, after 24 h and after 72 h of treatment, DNase-digested and transcribed to cDNA. A quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR green chemistry with subsequent melting curve analysis in technical triplicates. The 2-ddCt was calculated relative to the pre-determined housekeeping gene encoding succinate dehydrogenase complex subunit C ( SDHC ). (C) Baseline expression of the monocyte/macrophage markers CD11b (integrin subunit alpha M, ITGAM), CD11c (integrin subunit alpha X, ITGAX), and CD14, the granulocyte marker CD15 (fucosyltransferase 4, FUT4), and the general myeloid marker CD13 (aminopeptidase N, APN) in CASC15 -KO and control cells. The percentages of positive cells, quantified by flow cytometry after 72 h, are shown. (D-F) Growth rate and CD11c myeloid cell surface marker expression of CASC15 and control cell lines during drug-induced in vitro differentiation
- Conjugate
- Green dye
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry