Antibody data
- Antibody Data
- Antigen structure
- References [23]
- Comments [0]
- Validations
- Flow cytometry [2]
- Other assay [12]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-0116-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD11c Monoclonal Antibody (3.9), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The 3.9 monoclonal antibody reacts with human CD11c, the 150 kDa integrin alphaX chain. CD11c non-covalently associates with beta2 integrin to form the CD11c/CD18 heterodimer. This complex is expressed on monocytes, granulocytes, macrophages, NK, dendritic cells and subset of T and B lymphocytes. CD11c/CD18 binds to CD54, iC3b and fibrinogen and plays a role in leukocyte adhesive interactions. Applications Reported: The 3.9 antibody has been reported for use in flow cytometric analysis, and immunohistochemical staining of frozen tissue sections. Applications Tested: The 3.9 antibody has been tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at less than or equal to 1 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 3.9
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references Aluminum hydroxide adjuvant diverts the uptake and trafficking of genetically detoxified pertussis toxin to lysosomes in macrophages.
Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus.
Upregulation of CD3ζ and L-selectin in antigen-specific cytotoxic T lymphocytes by eliminating myeloid-derived suppressor cells with doxorubicin to improve killing efficacy of neuroblastoma cells in vitro.
Increased TNF-α Initiates Cytoplasmic Vacuolization in Whole Blood Coculture with Dengue Virus.
The long non-coding RNA Cancer Susceptibility 15 (CASC15) is induced by isocitrate dehydrogenase (IDH) mutations and maintains an immature phenotype in adult acute myeloid leukemia.
Human and Mouse Transcriptome Profiling Identifies Cross-Species Homology in Pulmonary and Lymph Node Mononuclear Phagocytes.
HBeAg seroconversion is associated with a more effective PD-L1 blockade during chronic hepatitis B infection.
AG490 reverses phenotypic alteration of dendritic cells by bladder cancer cells.
Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis.
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis.
Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia.
Brief Report: IFIH1 Mutation Causes Systemic Lupus Erythematosus With Selective IgA Deficiency.
Thymic HIV-2 infection uncovers posttranscriptional control of viral replication in human thymocytes.
Towards programming immune tolerance through geometric manipulation of phosphatidylserine.
Generation and in vivo evaluation of IL10-treated dendritic cells in a nonhuman primate model of AAV-based gene transfer.
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma.
DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues.
Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPα.
Effect of plasma viremia on apoptosis and immunophenotype of dendritic cells subsets in acute SIVmac239 infection of Chinese rhesus macaques.
Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis.
CD81 is essential for the formation of membrane protrusions and regulates Rac1-activation in adhesion-dependent immune cell migration.
Interferon-gamma promoter hypomethylation and increased expression in chronic periodontitis.
Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha.
Jaldin-Fincati J, Moussaoui S, Gimenez MC, Ho CY, Lancaster CE, Botelho R, Ausar F, Brookes R, Terebiznik M
Molecular microbiology 2022 May;117(5):1173-1195
Molecular microbiology 2022 May;117(5):1173-1195
Isolevuglandins disrupt PU.1-mediated C1q expression and promote autoimmunity and hypertension in systemic lupus erythematosus.
Patrick DM, de la Visitación N, Krishnan J, Chen W, Ormseth MJ, Stein CM, Davies SS, Amarnath V, Crofford LJ, Williams JM, Zhao S, Smart CD, Dikalov S, Dikalova A, Xiao L, Van Beusecum JP, Ao M, Fogo AB, Kirabo A, Harrison DG
JCI insight 2022 Jul 8;7(13)
JCI insight 2022 Jul 8;7(13)
Upregulation of CD3ζ and L-selectin in antigen-specific cytotoxic T lymphocytes by eliminating myeloid-derived suppressor cells with doxorubicin to improve killing efficacy of neuroblastoma cells in vitro.
Xu W, Li S, Li M, Zhou H, Yang X
Journal of clinical laboratory analysis 2022 Jan;36(1):e24158
Journal of clinical laboratory analysis 2022 Jan;36(1):e24158
Increased TNF-α Initiates Cytoplasmic Vacuolization in Whole Blood Coculture with Dengue Virus.
Satria RD, Huang TW, Jhan MK, Shen TJ, Tseng PC, Wang YT, Yang ZY, Hsing CH, Lin CF
Journal of immunology research 2021;2021:6654617
Journal of immunology research 2021;2021:6654617
The long non-coding RNA Cancer Susceptibility 15 (CASC15) is induced by isocitrate dehydrogenase (IDH) mutations and maintains an immature phenotype in adult acute myeloid leukemia.
Grasedieck S, Ruess C, Krowiorz K, Lux S, Pochert N, Schwarzer A, Klusmann JH, Jongen-Lavrencic M, Herold T, Bullinger L, Pollack JR, Rouhi A, Kuchenbauer F
Haematologica 2020 Sep 1;105(9):e448-453
Haematologica 2020 Sep 1;105(9):e448-453
Human and Mouse Transcriptome Profiling Identifies Cross-Species Homology in Pulmonary and Lymph Node Mononuclear Phagocytes.
Leach SM, Gibbings SL, Tewari AD, Atif SM, Vestal B, Danhorn T, Janssen WJ, Wager TD, Jakubzick CV
Cell reports 2020 Nov 3;33(5):108337
Cell reports 2020 Nov 3;33(5):108337
HBeAg seroconversion is associated with a more effective PD-L1 blockade during chronic hepatitis B infection.
Ferrando-Martinez S, Huang K, Bennett AS, Sterba P, Yu L, Suzich JA, Janssen HLA, Robbins SH
JHEP reports : innovation in hepatology 2019 Sep;1(3):170-178
JHEP reports : innovation in hepatology 2019 Sep;1(3):170-178
AG490 reverses phenotypic alteration of dendritic cells by bladder cancer cells.
Xiu W, Ma J, Lei T, Zhang M
Oncology letters 2018 Sep;16(3):2851-2856
Oncology letters 2018 Sep;16(3):2851-2856
Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis.
Liu B, Zhang M, Zhao J, Zheng M, Yang H
Experimental and therapeutic medicine 2018 Dec;16(6):5009-5014
Experimental and therapeutic medicine 2018 Dec;16(6):5009-5014
dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis.
Lim S, Kim WJ, Kim YH, Lee S, Koo JH, Lee JA, Yoon H, Kim DH, Park HJ, Kim HM, Lee HG, Yun Kim J, Lee JU, Hun Shin J, Kyun Kim L, Doh J, Kim H, Lee SK, Bothwell ALM, Suh M, Choi JM
Nature communications 2015 Sep 15;6:8244
Nature communications 2015 Sep 15;6:8244
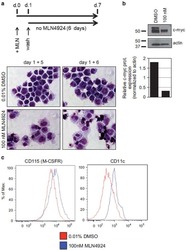
Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia.
Khalife J, Radomska HS, Santhanam R, Huang X, Neviani P, Saultz J, Wang H, Wu YZ, Alachkar H, Anghelina M, Dorrance A, Curfman J, Bloomfield CD, Medeiros BC, Perrotti D, Lee LJ, Lee RJ, Caligiuri MA, Pichiorri F, Croce CM, Garzon R, Guzman ML, Mendler JH, Marcucci G
Leukemia 2015 Oct;29(10):1981-92
Leukemia 2015 Oct;29(10):1981-92
Brief Report: IFIH1 Mutation Causes Systemic Lupus Erythematosus With Selective IgA Deficiency.
Van Eyck L, De Somer L, Pombal D, Bornschein S, Frans G, Humblet-Baron S, Moens L, de Zegher F, Bossuyt X, Wouters C, Liston A
Arthritis & rheumatology (Hoboken, N.J.) 2015 Jun;67(6):1592-7
Arthritis & rheumatology (Hoboken, N.J.) 2015 Jun;67(6):1592-7
Thymic HIV-2 infection uncovers posttranscriptional control of viral replication in human thymocytes.
Nunes-Cabaço H, Matoso P, Foxall RB, Tendeiro R, Pires AR, Carvalho T, Pinheiro AI, Soares RS, Sousa AE
Journal of virology 2015 Feb;89(4):2201-8
Journal of virology 2015 Feb;89(4):2201-8
Towards programming immune tolerance through geometric manipulation of phosphatidylserine.
Roberts RA, Eitas TK, Byrne JD, Johnson BM, Short PJ, McKinnon KP, Reisdorf S, Luft JC, DeSimone JM, Ting JP
Biomaterials 2015 Dec;72:1-10
Biomaterials 2015 Dec;72:1-10
Generation and in vivo evaluation of IL10-treated dendritic cells in a nonhuman primate model of AAV-based gene transfer.
Moreau A, Vandamme C, Segovia M, Devaux M, Guilbaud M, Tilly G, Jaulin N, Le Duff J, Cherel Y, Deschamps JY, Anegon I, Moullier P, Cuturi MC, Adjali O
Molecular therapy. Methods & clinical development 2014;1:14028
Molecular therapy. Methods & clinical development 2014;1:14028
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma.
Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD
Science translational medicine 2013 Feb 27;5(174):174ra26
Science translational medicine 2013 Feb 27;5(174):174ra26
DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues.
Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, Håkansson UK, Moita LF, Agace WW, Bonnet D, Reis e Sousa C
Blood 2012 Jun 21;119(25):6052-62
Blood 2012 Jun 21;119(25):6052-62
Targeting CDK1 promotes FLT3-activated acute myeloid leukemia differentiation through C/EBPα.
Radomska HS, Alberich-Jordà M, Will B, Gonzalez D, Delwel R, Tenen DG
The Journal of clinical investigation 2012 Aug;122(8):2955-66
The Journal of clinical investigation 2012 Aug;122(8):2955-66
Effect of plasma viremia on apoptosis and immunophenotype of dendritic cells subsets in acute SIVmac239 infection of Chinese rhesus macaques.
Xia HJ, Ma JP, Zhang GH, Han JB, Wang JH, Zheng YT
PloS one 2011;6(12):e29036
PloS one 2011;6(12):e29036
Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis.
Morbach H, Wiegering V, Richl P, Schwarz T, Suffa N, Eichhorn EM, Eyrich M, Girschick HJ
Arthritis and rheumatism 2011 Nov;63(11):3458-66
Arthritis and rheumatism 2011 Nov;63(11):3458-66
CD81 is essential for the formation of membrane protrusions and regulates Rac1-activation in adhesion-dependent immune cell migration.
Quast T, Eppler F, Semmling V, Schild C, Homsi Y, Levy S, Lang T, Kurts C, Kolanus W
Blood 2011 Aug 18;118(7):1818-27
Blood 2011 Aug 18;118(7):1818-27
Interferon-gamma promoter hypomethylation and increased expression in chronic periodontitis.
Zhang S, Crivello A, Offenbacher S, Moretti A, Paquette DW, Barros SP
Journal of clinical periodontology 2010 Nov;37(11):953-61
Journal of clinical periodontology 2010 Nov;37(11):953-61
Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha.
Jin JO, Park HY, Xu Q, Park JI, Zvyagintseva T, Stonik VA, Kwak JY
Blood 2009 Jun 4;113(23):5839-47
Blood 2009 Jun 4;113(23):5839-47
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
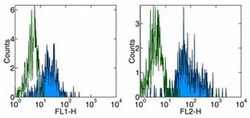
- Experimental details
- Staining of normal human peripheral blood cells with Anti-Human CD11c FITC (left) and PE (right). Appropriate isotype controls were used (open histogram). Cells in the monocyte population were used for analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
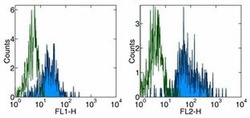
- Experimental details
- Staining of normal human peripheral blood cells with Anti-Human CD11c FITC (left) and PE (right). Appropriate isotype controls were used (open histogram). Cells in the monocyte population were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Immune profiling in DENV-treated whole blood cells 24 h postincubation. Following DENV (MOI = 1) coculture in 100 mu l of WB ex vivo for 24 h, (a) representative flow cytometric analysis and gating of various cells obtained from five cases, performed by staining for specific cell surface markers (CD4, CD8, CD11c, CD14, CD16, CD19, CD25, CD56, CD62L, and HLA-DR), in the DENV-infected and mock groups showed (b) the changes in the expression of specific immune cell populations as noted. (c) The results are shown as a percentage of the mean +- SD obtained from five cases. * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the mock group. R: region; WBC: white blood cell; bri: bright.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
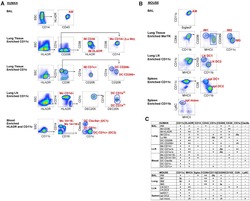
- Figure 1. Gating Strategy to Sort Human and Mouse Mononuclear Phagocytes for RNA Isolation (A and B) Fluorescence-activated cell sorting (FACS) gating strategy used to isolate human (A) or mouse (B) mononuclear phagocytes (MPs, labeled in red) from the indicated tissues. Before sorting, cell suspensions were magnetically enriched as indicated and gated to select Live; Single; CD45 + ; Lineage - cells as shown in Figures S1 and S2 . Arrows indicate where further analysis is performed on the specified subpopulation. The data presented are representative of 3-6 replicates per sort. (C) Table illustrating cell surface marker expression by MP subsets in human (top) and mouse (bottom). - or lo signifies no or little expression, + or ++ indicates expression or strong expression, -/+ indicates heterogeneous expression of marker proteins.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
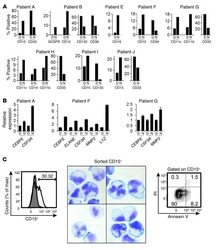
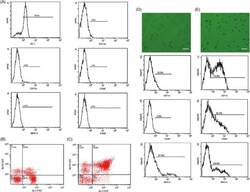
- FIGURE 1 Extraction, identification, and purification of myeloid-derived suppressor cell and cultivation of dendritic cell. (A) Cells were extracted from the bone marrow of BALB/c mice and stained by monoclonal antibodies. Under flow cytometry, the expressive rate of Gr-1 + MDSC, CD11b + MDSC, CD11c + MDSC, CD80 + MDSC, F4/80 + MDSC, and MHC-II + MDSC were 70.4%, 3.5%, 4.8%, 1.2%, 0.3%, 2.1% respectively. (B) The expressive rate of Gr-1 + CD11b + MDSC was 22.6%. (C) After MACS by CD11b magnetic bead, purification of Gr-1 + CD11b + MDSC reached 84.6%. (D) Most non-antigen-loaded dendritic cells grew adherently, with different sizes, star or spindle shape, and stretching tubers, but some of the cells seemed to have adopted a half-adherent state with rough surface. The expressive rates of CD11c, CD86, and MHC-II on DCs were 10.9%, 3.8%, and 27.9%, respectively, by flow cytometry. (E) On the 7th day, DCs were stimulated and activated by tumor antigens. DCs in the half-adherent state increased obviously with radial spikes and bigger shape. The expressive rates of CD11c, CD86, and MHC-II were 74.8%, 50.3%, and 49.8%, respectively, by flow cytometry. IgG FITC, a homotypic control antibody, was used to set the gate strategy. Scale bar = 100 mumol/liter. MDSC, myeloid-derived suppressor cell; MACS, magnetic-activated cell sorting; DC, dendritic cell; CD, cluster of differentiation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
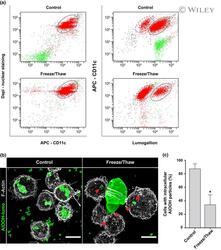
- 5 FIGURE Impact of particle size on aluminum adjuvant internalization. (a) U-937 cells were incubated with AlOOH-lumo (control) or AlOOH-lumo previously subjected to freeze/thaw cycles (freeze/thaw) to increase the size of the particles, as described in experimental procedures. The cells were incubated at 37degC for 4 h and subsequently processed for flow cytometry analysis. Differentiated macrophages were classified using anti-CD11c antibodies. Cell nuclei were labeled with DAPI. Cell populations encircled by a dotted line have not internalized AlOOH-Lumo and cells that have internalized AlOOH-Lumo are encircled by a solid line. (b) U-937 cells were incubated with control or freeze/thaw AlOOH-lumo preparations as described above and subsequently fixed and F-Actin stained with blue phalloidin (pseudocolored gray). Red arrowheads in the right panel point to small, internalized adjuvant particles. Spinning disk confocal images represent a merge of z-stacks. Images are representative of three independent trials. Fifty cells per trial per condition were analyzed. Scale bars, 5 mum. (c) U-937 cells were incubated with control or freeze/thaw AlOOH-lumo preparations and processed as described in (b). The percentage of cells with intracellular AlOOH particles were calculated for three independent trials and the mean represented in the bar graph. * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
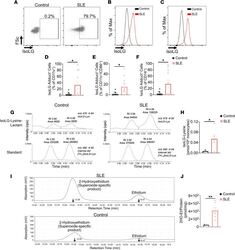
- IsoLG adducts are enriched in monocytes of patients with SLE. ( A ) Representative FACS plots displaying isoLG adduct containing CD11c + PBMCs from a representative control and patient with SLE. Representative histograms displaying the distribution of isoLG adducts in ( B ) CD11c + and ( C ) CD11c + CD86 + cells. Quantitation of IsoLG adduct-containing cells as a percentage of ( D ) CD11c + , ( E ) CD11c + CD86 + , and ( F ) CD14 + cells. For B - F data were analyzed using 1-tailed Student's t test or Mann-Whitney U test ( n = 10-11, *P < 0.05). ( G ) Stable isotope dilution multiple reaction monitoring for mass spectrometry analysis of isoLG-lysine-lactam adduct in DCs. Representative liquid chromatography/mass spectrometry chromatographs from a representative patient. The top row shows multiple reaction monitoring chromatographs for isoLG lysine lactam in samples, while the bottom row shows multiple reaction monitoring chromatograph for [13C615N2] internal standard for the same samples. cps, counts per second; Rt, retention time. ( H ) Quantitation of isoLG-lysine in monocytes from a subset of SLE patients and controls. ( I ) Monocytes from SLE patients and controls were sorted. Superoxide was detected using HPLC to monitor conversion of dihydroethidium to the superoxide oxidation adduct 2-hydroxyethidium (2-HO-ET) and ethidium. ( J ) Quantitation of 2-HO-ET from SLE patients and controls. For H and J , comparisons were made with a 1-tailed Student's t test ( n = 4-6, *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
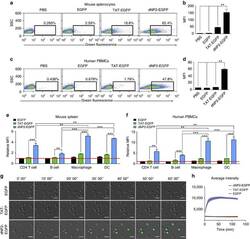
- Figure 2 Protein delivery efficiency of dNP2 in primary mouse and human immune cells. ( a , b ) Mouse primary splenocytes were isolated from 6-week-old female C57BL/6 mice and the cells were incubated with 5 muM EGFP, TAT- and dNP2-EGFP for 2 h. Intracellular fluorescence was analysed by flow cytometry and the data are represented as dot plots or mean fluorescence intensity (MFI) of the cells. ( c , d ) Human PBMCs were isolated from healthy donor blood and the cells were incubated with 5 muM EGFP, TAT-, dNP2-EGFP for 2 h. The data were analysed as described above. ( e ) Total splenocytes were incubated with 1 muM EGFP, TAT-, and dNP2-EGFP for 2 h. Cells were gated using markers specific for CD4 T cells (CD4 + ), B cells (CD19 + ), macrophages (CD11c lo CD11b hi F480 + ) and DCs (CD11c hi MHCII hi ). The EGFP signal in each cell population was then analysed by flow cytometric analysis. The relative MFI value was normalization to PBS treated cells. The red line indicates relative MFI of PBS-treated cells. ( f ) Total PBMCs were incubated with 1 muM EGFP, TAT-, and dNP2-EGFP for 2 h. Cells were gated with markers specific for CD4 T cells (CD4 + ), B cells (CD19 + ), macrophages (CD11b + ) and DCs (CD11c + ) and the data were then analysed as described above. ( g ) Time-lapse images of mouse CD4 T cells incubated with 1 muM EGFP, TAT- and dNP2-EGFP were acquired for 2 h (Scale bar, 15 mum) and ( h ) the average fluorescence intensities of 10 cells from each sample were calculate
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
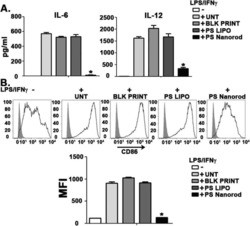
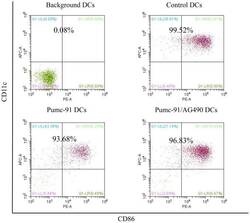
- Figure 2. Percentage of purified DC samples in all experiments. DCs were labeled with the Non-DC Depletion Cocktail for negative selection. Next, DCs were labeled with DC Enrichment Cocktail for positive selection. The expression of CD11c and CD86 was tested by flow cytometery. The percentages of purified DC are presented in the top of each panel. DCs, dendritic cells; CD, cluster of differentiation.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
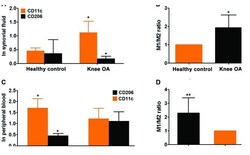
- Figure 2. Detection of surface markers CD11c and CD206 expression was carried out using flow cytometry approach in the synovial fluids biopsied and peripheral blood from participants. (A) Expression of CD11c and CD206 surface markers of M1 and M2 macrophages was evaluated using flow cytometry in the synovial fluids biopsied from 35 patients with knee OA and paired healthy control. (B) Analysis of ratio of M1 to M2 macrophages in healthy controls and knee OA. (C) Likewise, expression of CD11c and CD206 surface markers was assayed using flow cytometry in the peripheral blood sampled from 80 cases of patients with knee OA and paired healthy control. (D) Similarly, ratio of M1 to M2 macrophages was calculated in healthy controls and knee OA. In light of the data being non-normal distribution, Mann-Whiney U test was used to analyze the statistical difference between healthy control and knee OA. *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
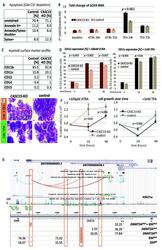
- Figure 1. CASC15 -KO promotes the differentiation of acute myeloid leukemia cells. (A) Apoptosis in CASC15 -KO and empty vector-transduced (control) OCI-AML5 cell lines after 24 h of depletion of granulocyte-macrophage colony-stimulating factor (annexin-FITC/Sytox blue flow cytometry). (B) Expression of SOX4 during in vitro differentiation of CASC15 -KO and control OCI-AML5 cell lines. All cells were treated with 0.1 mM all- trans retinoic acid (ATRA) and 1 nM 12-O-tetradecanoylphorbol-13-acetate (TPA) over 72 h in three independent experiments. Total RNA was extracted before, after 24 h and after 72 h of treatment, DNase-digested and transcribed to cDNA. A quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR green chemistry with subsequent melting curve analysis in technical triplicates. The 2-ddCt was calculated relative to the pre-determined housekeeping gene encoding succinate dehydrogenase complex subunit C ( SDHC ). (C) Baseline expression of the monocyte/macrophage markers CD11b (integrin subunit alpha M, ITGAM), CD11c (integrin subunit alpha X, ITGAX), and CD14, the granulocyte marker CD15 (fucosyltransferase 4, FUT4), and the general myeloid marker CD13 (aminopeptidase N, APN) in CASC15 -KO and control cells. The percentages of positive cells, quantified by flow cytometry after 72 h, are shown. (D-F) Growth rate and CD11c myeloid cell surface marker expression of CASC15 and control cell lines during drug-induced in vitro differentiation
 Explore
Explore Validate
Validate Learn
Learn Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry