Antibody data
- Antibody Data
- Antigen structure
- References [3]
- Comments [0]
- Validations
- Immunohistochemistry [1]
- Flow cytometry [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-19007 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD18 Monoclonal Antibody (MEM-48)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- This antibody recognizes an extracellular epitope involving residues 534-546 in cysteine-rich repeat 3 of the CD18 antigen (integrin beta2 subunit; beta2 integrin). CD18 is a 90-95 kDa type I transmembrane protein expressed on all leukocytes. It will not cross-react with canine. Endotoxin level is less than 0.01 EU/ug of the protein, as determined by the LAL test. Western Blot: non-reducing conditions; Functional Application: Induction.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- MEM-48
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- 4°C, do not freeze
Submitted references Searching for common stem cells of the hepatic and hematopoietic systems in the human fetal liver: CD34+ cytokeratin 7/8+ cells express markers for stellate cells.
Searching for common stem cells of the hepatic and hematopoietic systems in the human fetal liver: CD34+ cytokeratin 7/8+ cells express markers for stellate cells.
Increased phagocyte Fc gammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects.
Suskind DL, Muench MO
Journal of hepatology 2004 Feb;40(2):261-8
Journal of hepatology 2004 Feb;40(2):261-8
Searching for common stem cells of the hepatic and hematopoietic systems in the human fetal liver: CD34+ cytokeratin 7/8+ cells express markers for stellate cells.
Suskind DL, Muench MO
Journal of hepatology 2004 Feb;40(2):261-8
Journal of hepatology 2004 Feb;40(2):261-8
Increased phagocyte Fc gammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects.
Schiff DE, Rae J, Martin TR, Davis BH, Curnutte JT
Blood 1997 Oct 15;90(8):3187-94
Blood 1997 Oct 15;90(8):3187-94
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
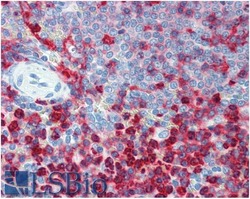
- Experimental details
- Immunohistochemistry staining of human spleen (paraffin sections) using anti-CD18 (MEM-48) Monoclonal antibody (Product # MA1-19007.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
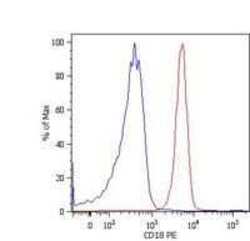
- Experimental details
- Flow cytometry analysis of CD18 using a monoclonal antibody (Product # MA1-19007).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
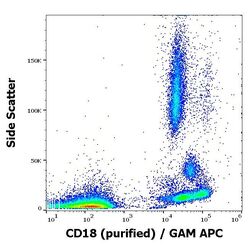
- Experimental details
- Flow cytometry surface staining pattern of human peripheral whole blood stained using anti-human CD18 (MEM-48) purifiedMonoclonal antibody (Product # MA1-19007) (concentration in sample 1 µg/mL, GAM APC).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
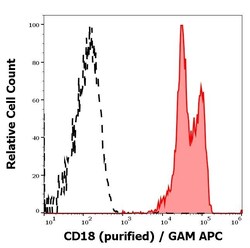
- Experimental details
- Separation of human leukocytes (red-filled) from CD18 negative blood debris (black-dashed) in flow cytometry analysis (surface staining) of human peripheral whole blood stained using anti-human CD18 (MEM-48) purified Monoclonal antibody (Product # MA1-19007) (concentration in sample 1 µg/mL, GAM APC).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
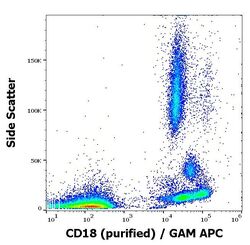
- Experimental details
- Flow Cytometry analysis of CD18 using CD18 Monoclonal Antibody (MEM-48) (Product # MA1-19007). Flow cytometry surface staining pattern of human peripheral whole blood stained using anti-human CD18 (MEM-48) purified antibody (concentration in sample 1 μg/mL, GAM APC).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
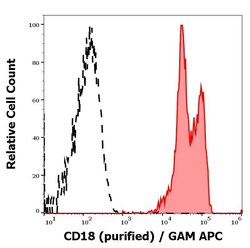
- Experimental details
- Flow Cytometry analysis of CD18 using CD18 Monoclonal Antibody (MEM-48) (Product # MA1-19007). Separation of human leukocytes (red-filled) from CD18 negative blood debris (black-dashed) in flow cytometry analysis (surface staining) of human peripheral whole blood stained using anti-human CD18 (MEM-48) purified antibody (concentration in sample 1 μg/mL, GAM APC).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry