Antibody data
- Antibody Data
- Antigen structure
- References [25]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [2]
- Flow cytometry [1]
- Other assay [10]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 32-0300 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- GluR2 Monoclonal Antibody (6C4)
- Antibody type
- Monoclonal
- Antigen
- Other
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 6C4
- Vial size
- 100 μg
- Concentration
- 0.5 mg/mL
- Storage
- -20°C
Submitted references Prickle promotes the formation and maintenance of glutamatergic synapses by stabilizing the intercellular planar cell polarity complex.
Role of Satb1 and Satb2 Transcription Factors in the Glutamate Receptors Expression and Ca(2+) Signaling in the Cortical Neurons In Vitro.
Trans-synaptic assemblies link synaptic vesicles and neuroreceptors.
Evidence that Brain-Reactive Autoantibodies Contribute to Chronic Neuronal Internalization of Exogenous Amyloid-β1-42 and Key Cell Surface Proteins During Alzheimer's Disease Pathogenesis.
Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions.
Sex differences in the role of atypical PKC within the basolateral nucleus of the amygdala in a mouse hyperalgesic priming model.
Glutamate Receptor Trafficking and Protein Synthesis Mediate the Facilitation of LTP by Secreted Amyloid Precursor Protein-Alpha.
The Temporal Dynamics of Arc Expression Regulate Cognitive Flexibility.
Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects.
NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation.
L-Stepholidine rescues memory deficit and synaptic plasticity in models of Alzheimer's disease via activating dopamine D1 receptor/PKA signaling pathway.
Cocaine-induced plasticity in the cerebellum of sensitised mice.
Redistribution of ionotropic glutamate receptors detected by laser microdissection of the rat dentate gyrus 48 h following LTP induction in vivo.
Rapid neurogenesis through transcriptional activation in human stem cells.
Rapid neurogenesis through transcriptional activation in human stem cells.
GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons.
Rapid visual stimulation increases extrasynaptic glutamate receptor expression but not visual-evoked potentials in the adult rat primary visual cortex.
Increased expression, but not postsynaptic localisation, of ionotropic glutamate receptors during the late-phase of long-term potentiation in the dentate gyrus in vivo.
Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test.
Learning-induced glutamate receptor phosphorylation resembles that induced by long term potentiation.
Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals.
A molecular mechanism for stabilization of learning-induced synaptic modifications.
Glutamate receptor subunit 3 (GluR3) immunoreactivity delineates a subpopulation of parvalbumin-containing interneurons in the rat hippocampus.
Glutamate receptor subunit GluR2 and NMDAR1 immunoreactivity in the retina of macaque monkeys with experimental glaucoma does not identify vulnerable neurons.
Structural determinants of ion flow through recombinant glutamate receptor channels.
Ban Y, Yu T, Feng B, Lorenz C, Wang X, Baker C, Zou Y
Science advances 2021 Oct 8;7(41):eabh2974
Science advances 2021 Oct 8;7(41):eabh2974
Role of Satb1 and Satb2 Transcription Factors in the Glutamate Receptors Expression and Ca(2+) Signaling in the Cortical Neurons In Vitro.
Turovsky EA, Turovskaya MV, Fedotova EI, Babaev AA, Tarabykin VS, Varlamova EG
International journal of molecular sciences 2021 May 31;22(11)
International journal of molecular sciences 2021 May 31;22(11)
Trans-synaptic assemblies link synaptic vesicles and neuroreceptors.
Martinez-Sanchez A, Laugks U, Kochovski Z, Papantoniou C, Zinzula L, Baumeister W, Lučić V
Science advances 2021 Mar;7(10)
Science advances 2021 Mar;7(10)
Evidence that Brain-Reactive Autoantibodies Contribute to Chronic Neuronal Internalization of Exogenous Amyloid-β1-42 and Key Cell Surface Proteins During Alzheimer's Disease Pathogenesis.
Goldwaser EL, Acharya NK, Wu H, Godsey GA, Sarkar A, DeMarshall CA, Kosciuk MC, Nagele RG
Journal of Alzheimer's disease : JAD 2020;74(1):345-361
Journal of Alzheimer's disease : JAD 2020;74(1):345-361
Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions.
Morise J, Yamamoto S, Midorikawa R, Takamiya K, Nonaka M, Takematsu H, Oka S
International journal of molecular sciences 2020 Jul 19;21(14)
International journal of molecular sciences 2020 Jul 19;21(14)
Sex differences in the role of atypical PKC within the basolateral nucleus of the amygdala in a mouse hyperalgesic priming model.
Baptista-de-Souza D, Tavares-Ferreira D, Megat S, Sankaranarayanan I, Shiers S, Flores CM, Ghosh S, Luiz Nunes-de-Souza R, Canto-de-Souza A, Price TJ
Neurobiology of pain (Cambridge, Mass.) 2020 Aug-Dec;8:100049
Neurobiology of pain (Cambridge, Mass.) 2020 Aug-Dec;8:100049
Glutamate Receptor Trafficking and Protein Synthesis Mediate the Facilitation of LTP by Secreted Amyloid Precursor Protein-Alpha.
Mockett BG, Guévremont D, Elder MK, Parfitt KD, Peppercorn K, Morrissey J, Singh A, Hintz TJ, Kochen L, Tom Dieck S, Schuman E, Tate WP, Williams JM, Abraham WC
The Journal of neuroscience : the official journal of the Society for Neuroscience 2019 Apr 24;39(17):3188-3203
The Journal of neuroscience : the official journal of the Society for Neuroscience 2019 Apr 24;39(17):3188-3203
The Temporal Dynamics of Arc Expression Regulate Cognitive Flexibility.
Wall MJ, Collins DR, Chery SL, Allen ZD, Pastuzyn ED, George AJ, Nikolova VD, Moy SS, Philpot BD, Shepherd JD, Müller J, Ehlers MD, Mabb AM, Corrêa SAL
Neuron 2018 Jun 27;98(6):1124-1132.e7
Neuron 2018 Jun 27;98(6):1124-1132.e7
Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects.
Zhou Y, Kaiser T, Monteiro P, Zhang X, Van der Goes MS, Wang D, Barak B, Zeng M, Li C, Lu C, Wells M, Amaya A, Nguyen S, Lewis M, Sanjana N, Zhou Y, Zhang M, Zhang F, Fu Z, Feng G
Neuron 2016 Jan 6;89(1):147-62
Neuron 2016 Jan 6;89(1):147-62
NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation.
Frank RA, Komiyama NH, Ryan TJ, Zhu F, O'Dell TJ, Grant SG
Nature communications 2016 Apr 27;7:11264
Nature communications 2016 Apr 27;7:11264
L-Stepholidine rescues memory deficit and synaptic plasticity in models of Alzheimer's disease via activating dopamine D1 receptor/PKA signaling pathway.
Hao JR, Sun N, Lei L, Li XY, Yao B, Sun K, Hu R, Zhang X, Shi XD, Gao C
Cell death & disease 2015 Nov 5;6(11):e1965
Cell death & disease 2015 Nov 5;6(11):e1965
Cocaine-induced plasticity in the cerebellum of sensitised mice.
Vazquez-Sanroman D, Carbo-Gas M, Leto K, Cerezo-Garcia M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Rossi F, Miquel M
Psychopharmacology 2015 Dec;232(24):4455-67
Psychopharmacology 2015 Dec;232(24):4455-67
Redistribution of ionotropic glutamate receptors detected by laser microdissection of the rat dentate gyrus 48 h following LTP induction in vivo.
Kennard JT, Guévremont D, Mason-Parker SE, Abraham WC, Williams JM
PloS one 2014;9(3):e92972
PloS one 2014;9(3):e92972
Rapid neurogenesis through transcriptional activation in human stem cells.
Busskamp V, Lewis NE, Guye P, Ng AH, Shipman SL, Byrne SM, Sanjana NE, Murn J, Li Y, Li S, Stadler M, Weiss R, Church GM
Molecular systems biology 2014 Nov 17;10(11):760
Molecular systems biology 2014 Nov 17;10(11):760
Rapid neurogenesis through transcriptional activation in human stem cells.
Busskamp V, Lewis NE, Guye P, Ng AH, Shipman SL, Byrne SM, Sanjana NE, Murn J, Li Y, Li S, Stadler M, Weiss R, Church GM
Molecular systems biology 2014 Nov 17;10:760
Molecular systems biology 2014 Nov 17;10:760
GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons.
Ho VM, Dallalzadeh LO, Karathanasis N, Keles MF, Vangala S, Grogan T, Poirazi P, Martin KC
Molecular and cellular neurosciences 2014 Jul;61:1-12
Molecular and cellular neurosciences 2014 Jul;61:1-12
Rapid visual stimulation increases extrasynaptic glutamate receptor expression but not visual-evoked potentials in the adult rat primary visual cortex.
Eckert MJ, Guévremont D, Williams JM, Abraham WC
The European journal of neuroscience 2013 Feb;37(3):400-6
The European journal of neuroscience 2013 Feb;37(3):400-6
Increased expression, but not postsynaptic localisation, of ionotropic glutamate receptors during the late-phase of long-term potentiation in the dentate gyrus in vivo.
Kennard JT, Guévremont D, Mason-Parker SE, Abraham WC, Williams JM
Neuropharmacology 2009 Jan;56(1):66-72
Neuropharmacology 2009 Jan;56(1):66-72
Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test.
Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK
Neuropharmacology 2008 Mar;54(3):577-87
Neuropharmacology 2008 Mar;54(3):577-87
Learning-induced glutamate receptor phosphorylation resembles that induced by long term potentiation.
Shukla K, Kim J, Blundell J, Powell CM
The Journal of biological chemistry 2007 Jun 22;282(25):18100-18107
The Journal of biological chemistry 2007 Jun 22;282(25):18100-18107
Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals.
Williams JM, Guévremont D, Mason-Parker SE, Luxmanan C, Tate WP, Abraham WC
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Dec 19;27(51):14171-8
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Dec 19;27(51):14171-8
A molecular mechanism for stabilization of learning-induced synaptic modifications.
Quinlan EM, Lebel D, Brosh I, Barkai E
Neuron 2004 Jan 22;41(2):185-92
Neuron 2004 Jan 22;41(2):185-92
Glutamate receptor subunit 3 (GluR3) immunoreactivity delineates a subpopulation of parvalbumin-containing interneurons in the rat hippocampus.
Moga DE, Janssen WG, Vissavajjhala P, Czelusniak SM, Moran TM, Hof PR, Morrison JH
The Journal of comparative neurology 2003 Jul 14;462(1):15-28
The Journal of comparative neurology 2003 Jul 14;462(1):15-28
Glutamate receptor subunit GluR2 and NMDAR1 immunoreactivity in the retina of macaque monkeys with experimental glaucoma does not identify vulnerable neurons.
Hof PR, Lee PY, Yeung G, Wang RF, Podos SM, Morrison JH
Experimental neurology 1998 Oct;153(2):234-41
Experimental neurology 1998 Oct;153(2):234-41
Structural determinants of ion flow through recombinant glutamate receptor channels.
Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B
Science (New York, N.Y.) 1991 Jun 21;252(5013):1715-8
Science (New York, N.Y.) 1991 Jun 21;252(5013):1715-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
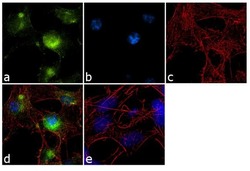
- Experimental details
- Immunofluorescence analysis of GluR2 was performed using 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with GluR2 (6C4) Mouse Monoclonal Antibody (Product # 32-0300) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
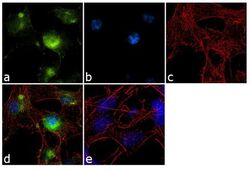
- Experimental details
- Immunofluorescence analysis of GluR2 was performed using 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with GluR2 (6C4) Mouse Monoclonal Antibody (Product # 32-0300) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e shows a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
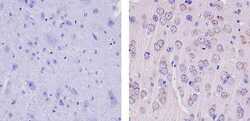
- Experimental details
- Immunohistochemistry analysis of GLUR2 showing staining in the membrane and weak staining in the cytoplasm of paraffin-embedded mouse brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a GLUR2 Mouse Monoclonal Antibody (Product # 32-0300) diluted in 3% BSA-PBS at a dilution of 1:100 for 1 hour at 37ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
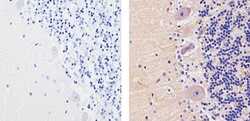
- Experimental details
- Immunohistochemistry analysis of GLUR2 showing staining in the cytoplasm of paraffin-embedded human cerebellum tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a GLUR2 Mouse Monoclonal Antibody (Product # 32-0300) diluted in 3% BSA-PBS at a dilution of 1:20 for 1 hour at 37ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
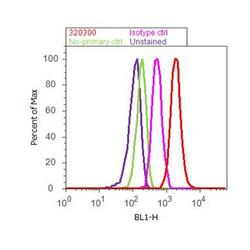
- Flow cytometry analysis of GluR2 was done on U-87 MG cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with GluR2 Mouse Monoclonal Antibody (320300, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
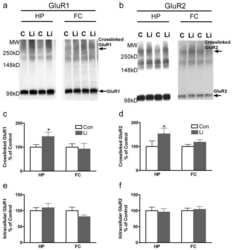
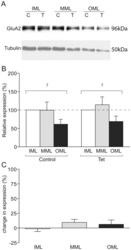
- Figure 5 GluA2 expression in LTP-stimulated rats. (a) Representative Western blot showing GluA2 (upper) and tubulin (lower) expression in laser microdissected tissue from one LTP-stimulated animal. (b) Mean relative expression of GluA2 in the molecular layer zones from LTP-stimulated and naive hemispheres, determined as a proportion of the ipsilateral inner molecular layer. (c) Mean percentage difference in tubulin-normalised expression of GluA2 in molecular layer zones of the LTP-stimulated hemisphere compared to the naive hemisphere; + p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
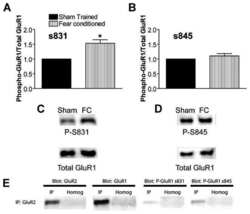
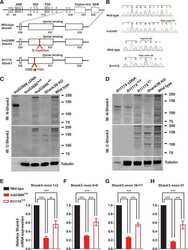
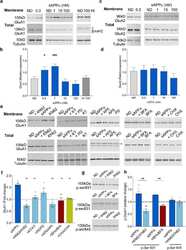
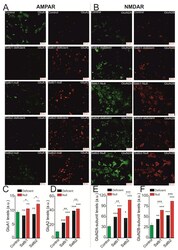
- Figure 3 Expression of subunits forming AMPAR (GluA1, GluA2) and NMDAR (GluN2A, GluN2B) in neurons of the cerebral cortex derived from Satb-deficient, Satb-, and control mice. ( A , B )--Immunostaining of GluA1 (green color), GluA2 (red color) subunits of AMPA receptors ( A ) and GluN2A (green color), GluN2B (red color) of NMDA receptors ( B ) of cortical neurons from control (Satb +/+ * Nex Cre/+ ), Satb-deficient (Satb fl/+ * Nex Cre/+ ) and Satb- (Satb2 fl/fl * Nex Cre/+ ) mice. ( C - F) --The effects of Satb- and Satb2-deletions on the level of GluA1 ( C ), GluA2 ( D ), GluN2A ( E ), and GluN2B ( F ) subunits. Intensity levels of surface-expressed receptor subunits were determined by confocal imaging. We analyzed individual neurons which had fluorescence of Alexa Fluor 633 (GluA1, green color), Alexa Fluor 488 (GluA2, red color) and Alexa Fluor-555 (GluN2B, red color), and Alexa Fluor-594 (GluN2A, green color). The quantitative data reflecting the level of subunits expression are presented as fluorescence intensity values in summary bar charts (mean +- SEM). The values were averaged by 300 +- 50 neurons for each column. Statistical significance was assessed using paired t-test. The results obtained after immunostaining well agree with the data of fluorescent Ca 2+ measurements presented in Figure 4 and Figure 5 . After Ca 2+ measurements, the cells were fixed and stained by the antibodies. We used the scans from three independent view fields for each experimental
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
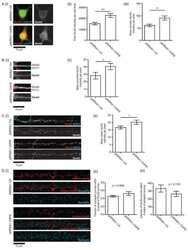
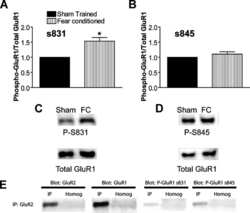
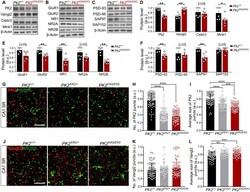
- Fig. 4. Altered composition of synaptic proteins in the PK2 E8Q/E8Q mice. ( A to C ) Western blot analysis of PCP components, regulator of PCP signaling, Mink1, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR)/N-methyl-D-aspartate receptor (NMDAR) subunits, and MAGUK family proteins in the crude synaptosomes of cortex and HPC from P14 PK2 E8Q/E8Q mice and WT littermates. ( D to F ) Quantitation of protein levels shown in (A) to (C). Each data point represents one animal. Data were normalized by dividing each value to the maximum value in each independent experiment. PK2 +/+ , n = 6; PK2 E8Q/E8Q , n = 6, Mann-Whitney U test, * P < 0.05 and ** P < 0.01. Error bars represent SEM. ( G to L ) Immunofluorescence staining of PK2 and Vangl2 in CA1 SR of P14 mice. To quantify the number (H) and the average size (I) of PK2 puncta, 66 image stacks of CA1 SR were taken from three PK2 +/+ animals, 66 image stacks from three PK2 E8Q/+ animals, and 68 image stacks from three PK2 E8Q/E8Q animals. To quantify the number (K) and the average size (L) of Vangl2 puncta, 62 image stacks were taken from three PK2 +/+ animals, 36 image stacks from two PK2 E8Q/+ animals, and 63 image stacks from PK2 E8Q/E8Q animals. Data were normalized by dividing each value to the maximum value in each independent experiment. Scale bars, 5 mum. Kruskal-Wallis test was used to compare the means of three groups, and Dunn's test was used to perform multiple comparisons. ** P < 0.01, *** P < 0
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry