434200
antibody from Invitrogen Antibodies
Targeting: NR5A1
AD4BP, ELP, FTZ1, FTZF1, hSF-1, SF-1, SF1
Antibody data
- Antibody Data
- Antigen structure
- References [15]
- Comments [0]
- Validations
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 434200 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- NR5A1 Monoclonal Antibody (N1665)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- This antibody specifically recognizes human SF-1 and cross reacts with mouse and rat SF-1.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- N1665
- Vial size
- 100 μL
- Concentration
- 1 mg/mL
- Storage
- Maintain refrigerated at 2-8°C for up to 1 month. For long term storage store at -20°C
Submitted references Establishment of a pure culture of immature Sertoli cells by PDGFRA staining and cell sorting.
Pre- and postoperative (68) Ga-DOTATOC positron emission tomography for hormone-secreting pituitary neuroendocrine tumours.
Pituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells.
Gonadotroph tumours with a low SF-1 labelling index are more likely to recur and are associated with enrichment of the PI3K-AKT pathway.
Digital analysis of hormonal immunostaining in pituitary adenomas classified according to WHO 2017 criteria and correlation with preoperative laboratory findings.
Essential and sex-specific effects of mGluR5 in ventromedial hypothalamus regulating estrogen signaling and glucose balance.
Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo.
Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies.
MAPK pathway control of stem cell proliferation and differentiation in the embryonic pituitary provides insights into the pathogenesis of papillary craniopharyngioma.
A genomic atlas of human adrenal and gonad development.
Oncogene-Induced Senescence in Pituitary Adenomas--an Immunohistochemical Study.
Steroidogenic factor 1 promotes aggressive growth of castration-resistant prostate cancer cells by stimulating steroid synthesis and cell proliferation.
Ovarian Brenner tumour: a morphologic and immunohistochemical analysis suggesting an origin from fallopian tube epithelium.
Elevated levels of the steroidogenic factor 1 are associated with over-expression of CYP19 in an oestrogen-producing testicular Leydig cell tumour.
ChIP-on-chip analysis reveals angiopoietin 2 (Ang2, ANGPT2) as a novel target of steroidogenic factor-1 (SF-1, NR5A1) in the human adrenal gland.
Malolina EA, Galiakberova AA, Dashinimaev EB, Kulibin AY
Molecular reproduction and development 2022 May;89(5-6):243-255
Molecular reproduction and development 2022 May;89(5-6):243-255
Pre- and postoperative (68) Ga-DOTATOC positron emission tomography for hormone-secreting pituitary neuroendocrine tumours.
Tjörnstrand A, Casar-Borota O, Heurling K, Schöll M, Gjertsson P, Ragnarsson O, Filipsson Nyström H
Clinical endocrinology 2021 Jun;94(6):956-967
Clinical endocrinology 2021 Jun;94(6):956-967
Pituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells.
Russell JP, Lim X, Santambrogio A, Yianni V, Kemkem Y, Wang B, Fish M, Haston S, Grabek A, Hallang S, Lodge EJ, Patist AL, Schedl A, Mollard P, Nusse R, Andoniadou CL
eLife 2021 Jan 5;10
eLife 2021 Jan 5;10
Gonadotroph tumours with a low SF-1 labelling index are more likely to recur and are associated with enrichment of the PI3K-AKT pathway.
Hickman RA, Bruce JN, Otten M, Khandji AG, Flowers XE, Siegelin M, Lopes B, Faust PL, Freda PU
Neuropathology and applied neurobiology 2021 Apr;47(3):415-427
Neuropathology and applied neurobiology 2021 Apr;47(3):415-427
Digital analysis of hormonal immunostaining in pituitary adenomas classified according to WHO 2017 criteria and correlation with preoperative laboratory findings.
Tamanini JVG, Dal Fabbro M, de Freitas LLL, Vassallo J, de Souza Queiroz L, Rogerio F
Neurosurgical focus 2020 Jun;48(6):E12
Neurosurgical focus 2020 Jun;48(6):E12
Essential and sex-specific effects of mGluR5 in ventromedial hypothalamus regulating estrogen signaling and glucose balance.
Fagan MP, Ameroso D, Meng A, Rock A, Maguire J, Rios M
Proceedings of the National Academy of Sciences of the United States of America 2020 Aug 11;117(32):19566-19577
Proceedings of the National Academy of Sciences of the United States of America 2020 Aug 11;117(32):19566-19577
Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo.
Janjic MM, Prévide RM, Fletcher PA, Sherman A, Smiljanic K, Abebe D, Bjelobaba I, Stojilkovic SS
Scientific reports 2019 Dec 27;9(1):20098
Scientific reports 2019 Dec 27;9(1):20098
Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies.
Portnoi MF, Dumargne MC, Rojo S, Witchel SF, Duncan AJ, Eozenou C, Bignon-Topalovic J, Yatsenko SA, Rajkovic A, Reyes-Mugica M, Almstrup K, Fusee L, Srivastava Y, Chantot-Bastaraud S, Hyon C, Louis-Sylvestre C, Validire P, de Malleray Pichard C, Ravel C, Christin-Maitre S, Brauner R, Rossetti R, Persani L, Charreau EH, Dain L, Chiauzzi VA, Mazen I, Rouba H, Schluth-Bolard C, MacGowan S, McLean WHI, Patin E, Rajpert-De Meyts E, Jauch R, Achermann JC, Siffroi JP, McElreavey K, Bashamboo A
Human molecular genetics 2018 Apr 1;27(7):1228-1240
Human molecular genetics 2018 Apr 1;27(7):1228-1240
MAPK pathway control of stem cell proliferation and differentiation in the embryonic pituitary provides insights into the pathogenesis of papillary craniopharyngioma.
Haston S, Pozzi S, Carreno G, Manshaei S, Panousopoulos L, Gonzalez-Meljem JM, Apps JR, Virasami A, Thavaraj S, Gutteridge A, Forshew T, Marais R, Brandner S, Jacques TS, Andoniadou CL, Martinez-Barbera JP
Development (Cambridge, England) 2017 Jun 15;144(12):2141-2152
Development (Cambridge, England) 2017 Jun 15;144(12):2141-2152
A genomic atlas of human adrenal and gonad development.
Del Valle I, Buonocore F, Duncan AJ, Lin L, Barenco M, Parnaik R, Shah S, Hubank M, Gerrelli D, Achermann JC
Wellcome open research 2017 Apr 7;2:25
Wellcome open research 2017 Apr 7;2:25
Oncogene-Induced Senescence in Pituitary Adenomas--an Immunohistochemical Study.
Manojlovic-Gacic E, Skender-Gazibara M, Popovic V, Soldatovic I, Boricic N, Raicevic S, Pekic S, Doknic M, Miljic D, Alafuzoff I, Pontén F, Casar-Borota O
Endocrine pathology 2016 Mar;27(1):1-11
Endocrine pathology 2016 Mar;27(1):1-11
Steroidogenic factor 1 promotes aggressive growth of castration-resistant prostate cancer cells by stimulating steroid synthesis and cell proliferation.
Lewis SR, Hedman CJ, Ziegler T, Ricke WA, Jorgensen JS
Endocrinology 2014 Feb;155(2):358-69
Endocrinology 2014 Feb;155(2):358-69
Ovarian Brenner tumour: a morphologic and immunohistochemical analysis suggesting an origin from fallopian tube epithelium.
Kuhn E, Ayhan A, Shih IeM, Seidman JD, Kurman RJ
European journal of cancer (Oxford, England : 1990) 2013 Dec;49(18):3839-49
European journal of cancer (Oxford, England : 1990) 2013 Dec;49(18):3839-49
Elevated levels of the steroidogenic factor 1 are associated with over-expression of CYP19 in an oestrogen-producing testicular Leydig cell tumour.
Straume AH, Løvås K, Miletic H, Gravdal K, Lønning PE, Knappskog S
European journal of endocrinology 2012 May;166(5):941-9
European journal of endocrinology 2012 May;166(5):941-9
ChIP-on-chip analysis reveals angiopoietin 2 (Ang2, ANGPT2) as a novel target of steroidogenic factor-1 (SF-1, NR5A1) in the human adrenal gland.
Ferraz-de-Souza B, Lin L, Shah S, Jina N, Hubank M, Dattani MT, Achermann JC
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011 Apr;25(4):1166-75
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011 Apr;25(4):1166-75
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
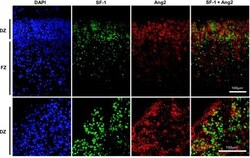
- Figure 5. SF-1 and Ang2 expression during early human fetal adrenal development. Top panel: immunohistochemistry for SF-1 and Ang2 in the human fetal adrenal gland (fetal stage 1, 8 wk postconception) shows strongest expression of these factors in the developing definitive zone (DZ). FZ, fetal zone. Nuclear staining is shown with DAPI. Bottom panel: higher-power magnification shows coexpression of SF-1 and Ang2 in areas of the definitive zone.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
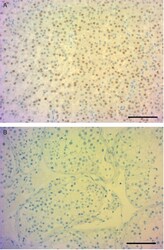
- Figure 6 Anti-SF-1 staining of tumour and normal tissue. Anti-SF-1-stained sections showed strong nuclear staining in tumour tissue (A) and negative staining in normal tissue (B). Scale bar: 100 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
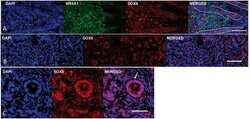
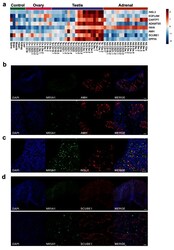
- Figure 13. Identification of potential testis-secreted proteins. ( a ) Heatmap representing normalised gene expression values for potential secreted proteins. The intensity of gene expression is indicated by a colour scale: blue for lowest and red for highest expression levels. ( b ) Immunohistochemistry for AMH (MIS) in human fetal testis at 9 wpc. NR5A1 (SF-1) was used to highlight Leydig cells (green). DAPI was used to counterstain nuclei (blue) and to highlight the outer capsule. Scale bars, 100 um (top panels) and 20 um (bottom panels) ( c ) Immunohistochemistry for INSL3 in human fetal testis at 9 wpc. NR5A1 (SF-1) was used to highlight Leydig cells (green). DAPI was used to counterstain nuclei (blue). Scale bar, 50 um ( d ) Immunohistochemistry for SCUBE1 in human fetal testis at 9 wpc. NR5A1 (SF-1) was used to highlight Leydig cells (green). DAPI was used to counterstain nuclei (blue). Scale bars, 100 um (top panels) and 20 um (bottom panels).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
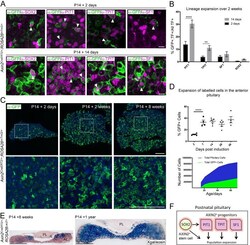
- Figure 1. Axin2 expressing cells contribute to pituitary growth and expansion of all lineages. ( A ) Immunofluorescence staining against GFP (green) with markers of pituitary stem cells (PSCs) or lineage commitment (magenta) in Axin2 CreERT2/+ ; ROSA26 mTmG/+ pituitaries harvested from mice induced at P14 and lineage traced for 2 days (top panel) and 14 days (bottom panel). Scale bar: 10 mum. ( B ) Quantification of lineage expansion between 2 and 14 days following induction at P14. Graph shows that the proportion of lineage committed cells (either PIT1 + , TPIT + , or SF1 + ) and PSCs (SOX2 + ), that is, that are transcription factor (TF) + cells that are GFP + increases between 2 days (black bars) and 14 days (grey bars) post-induction. PIT1 p=0.000004, TPIT p=0.008 multiple t -tests. n = 4 animals per time point. ( C ) Immunofluorescence staining against GFP (green) in pituitaries harvested from Axin2 CreERT2/+ ;ROSA26 mTmG/+ mice induced at P14 and lineage traced for 2 days, 2 weeks, and 8 weeks. Bottom panel shows magnified fields of view of regions of interest indicated by white boxes in panels above. Scale bars: 50 mum. ( D ) Top panel showing the quantification of the proportion of all cells in Axin2 CreERT2/+ ;ROSA26 mTmG/+ pituitaries that are GFP + at 2, 7, 14, 28, and 56 days post-induction as analysed by flow cytometry. Days 2-7 p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
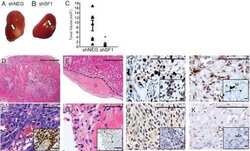
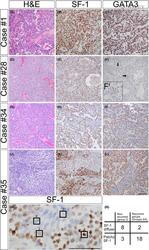
- 1 Figure Histological and immunohistochemical features of selected GT. A-C, Case #1 (Group 1) shows a monomorphic tumour with diffuse, strong SF-1 and GATA3 immunoreactivity. D-F, Case #28 (Group 2) was known not to recur within 8.6 years. The tumour is histologically monomorphic (D) and shows diffuse, strong SF-1 expression throughout the tumour (E). F, Unlike case #1, this tumour has rare neoplastic nuclei that weakly label for GATA3, shown at higher magnification in inset (F'). G-I, Case #34 (Group 3) recurred within 3.8 years after initial resection. SF-1 shows immunopositivity in a perivascular distribution with abrupt negative staining in the nests (H). A similar patchy staining is noted with a GATA3 immunostain (I). J-M, Case #35 (Group 3) recurred within 6.0 years. Clear cell change is noted in the centre of nests, whereas tumour cells with amphophilic cytoplasm are noted in a perivascular distribution. Patchy SF-1 (K) and GATA3 (L) staining is seen in the tumour. M: Immunocytologic variability of SF-1 staining within GT is shown and graded from 1-4. N: A 2 x 2 contingency table of Groups 2-4 showing that most recurrent tumours had patchy SF-1 labelling unlike in the non-recurrent group. Scale bar: 100 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
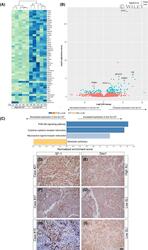
- 3 Figure Gene expression profiling data obtained following RNA sequencing. A, Heatmap of unsupervised hierarchical cluster analysis of the 50 most significantly differentially expressed genes after variance stabilising transformation of high SLI (>80%, n = 5) and low SLI (
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
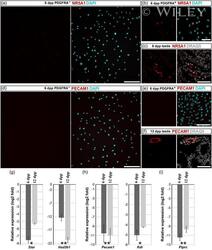
- 4 Figure The expression of marker genes for interstitial cells in 3-day cultures established from PDGFRA - and PDGFRA + cell populations. (a-c) Immunofluorescent staining of 6 dpp PDGFRA - (a), 6 dpp PDGFRA + (b) cultures and a testis section (c) for NR5A1, which is highly expressed in Leydig cells. (d-f) Immunofluorescent staining of 6 dpp PDGFRA - (d), 6 dpp PDGFRA + (e) cultures, and a testis section (f) for PECAM1, an endothelial cell marker. Right panels in (a-f) show merged images with DAPI- or DRAQ5-stained nuclei. (g-i) The expression levels of marker genes for Leydig cells (g), endothelial cells (h), and leukocytes (i) were low in 6 and 12 dpp PDGFRA - cultures relative to that in adult testicular tissue (the zero line). Data are presented as the mean +- SEM from three independent experiments. * p < 0.05 and ** p < 0.01. Scale bars: 200 mum (a,d) and 50 mum (b,c,e,f). dpp, days postpartum; PDGFR, platelet-derived growth factor receptor alpha and beta.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Other assay
Other assay