Antibody data
- Antibody Data
- Antigen structure
- References [27]
- Comments [0]
- Validations
- Immunocytochemistry [14]
- Immunohistochemistry [4]
- Flow cytometry [7]
- Other assay [12]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-110 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Nestin Monoclonal Antibody (10C2)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- MA1-110 has been successfully utilized in Western Blot, immunocytochemistry, flow cytometry, and immunofluorescence applications in human samples. MA1-110 also acts a neuronal stem cell marker and detects a band in Western blot at ~250 kDa.
- Reactivity
- Human, Mouse
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 10C2
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C
Submitted references EGF signaling promotes the lineage conversion of astrocytes into oligodendrocytes.
Adenomatoid odontogenic tumor: evidence for a mixed odontogenic tumor.
Survivin Inhibition by Piperine Sensitizes Glioblastoma Cancer Stem Cells and Leads to Better Drug Response.
Aberrant upregulation of the glycolytic enzyme PFKFB3 in CLN7 neuronal ceroid lipofuscinosis.
G-quadruplex DNA structures in human stem cells and differentiation.
Refining the Role of Pyruvate Dehydrogenase Kinases in Glioblastoma Development.
Primary cilia and SHH signaling impairments in human and mouse models of Parkinson's disease.
Suppression of premature transcription termination leads to reduced mRNA isoform diversity and neurodegeneration.
CPNE7-Induced Autophagy Restores the Physiological Function of Mature Odontoblasts.
Novel STMN2 Variant Linked to Amyotrophic Lateral Sclerosis Risk and Clinical Phenotype.
3D Visualization of Dynamic Cellular Reaction of Pulpal CD11c+ Dendritic Cells against Pulpitis in Whole Murine Tooth.
Glycoproteomic analysis of the changes in protein N-glycosylation during neuronal differentiation in human-induced pluripotent stem cells and derived neuronal cells.
SIRT1 regulates sphingolipid metabolism and neural differentiation of mouse embryonic stem cells through c-Myc-SMPDL3B.
microRNAs (miR 9, 124, 155 and 224) transdifferentiate mouse macrophages to neurons.
Non-invasive and high-throughput interrogation of exon-specific isoform expression.
An epilepsy-associated ACTL6B variant captures neuronal hyperexcitability in a human induced pluripotent stem cell model.
Reproducible differentiation and characterization of neurons from mouse embryonic stem cells.
In vitro human stem cell derived cultures to monitor calcium signaling in neuronal development and function.
A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids.
Transcriptional signatures of participant-derived neural progenitor cells and neurons implicate altered Wnt signaling in Phelan-McDermid syndrome and autism.
Vimentin Coordinates Protein Turnover at the Aggresome during Neural Stem Cell Quiescence Exit.
Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery.
Comparison of in vitro Neuronal Differentiation Capacity Between Mouse Epiblast Stem Cells Derived From Nuclear Transfer and Naturally Fertilized Embryos.
Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function.
Comparative immunomorphology of testicular Sertoli and sertoliform tumors.
Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma.
Androgen receptors and gender-specific distribution of alkaline phosphatase in human thyroid cartilage.
Liu X, Li C, Li J, Xie L, Hong Z, Zheng K, Zhao X, Yang A, Xu X, Tao H, Qiu M, Yang J
Molecular medicine (Cambridge, Mass.) 2022 May 4;28(1):50
Molecular medicine (Cambridge, Mass.) 2022 May 4;28(1):50
Adenomatoid odontogenic tumor: evidence for a mixed odontogenic tumor.
Barnts K, Feng JQ, Qin C, Zhang H, Cheng YL
Oral surgery, oral medicine, oral pathology and oral radiology 2022 Jun;133(6):675-683
Oral surgery, oral medicine, oral pathology and oral radiology 2022 Jun;133(6):675-683
Survivin Inhibition by Piperine Sensitizes Glioblastoma Cancer Stem Cells and Leads to Better Drug Response.
Warrier NM, Krishnan RK, Prabhu V, Hariharapura RC, Agarwal P, Kumar P
International journal of molecular sciences 2022 Jul 9;23(14)
International journal of molecular sciences 2022 Jul 9;23(14)
Aberrant upregulation of the glycolytic enzyme PFKFB3 in CLN7 neuronal ceroid lipofuscinosis.
Lopez-Fabuel I, Garcia-Macia M, Buondelmonte C, Burmistrova O, Bonora N, Alonso-Batan P, Morant-Ferrando B, Vicente-Gutierrez C, Jimenez-Blasco D, Quintana-Cabrera R, Fernandez E, Llop J, Ramos-Cabrer P, Sharaireh A, Guevara-Ferrer M, Fitzpatrick L, Thompton CD, McKay TR, Storch S, Medina DL, Mole SE, Fedichev PO, Almeida A, Bolaños JP
Nature communications 2022 Jan 27;13(1):536
Nature communications 2022 Jan 27;13(1):536
G-quadruplex DNA structures in human stem cells and differentiation.
Zyner KG, Simeone A, Flynn SM, Doyle C, Marsico G, Adhikari S, Portella G, Tannahill D, Balasubramanian S
Nature communications 2022 Jan 10;13(1):142
Nature communications 2022 Jan 10;13(1):142
Refining the Role of Pyruvate Dehydrogenase Kinases in Glioblastoma Development.
Larrieu CM, Storevik S, Guyon J, Pagano Zottola AC, Bouchez CL, Derieppe MA, Tan TZ, Miletic H, Lorens J, Tronstad KJ, Daubon T, Røsland GV
Cancers 2022 Aug 2;14(15)
Cancers 2022 Aug 2;14(15)
Primary cilia and SHH signaling impairments in human and mouse models of Parkinson's disease.
Schmidt S, Luecken MD, Trümbach D, Hembach S, Niedermeier KM, Wenck N, Pflügler K, Stautner C, Böttcher A, Lickert H, Ramirez-Suastegui C, Ahmad R, Ziller MJ, Fitzgerald JC, Ruf V, van de Berg WDJ, Jonker AJ, Gasser T, Winner B, Winkler J, Vogt Weisenhorn DM, Giesert F, Theis FJ, Wurst W
Nature communications 2022 Aug 16;13(1):4819
Nature communications 2022 Aug 16;13(1):4819
Suppression of premature transcription termination leads to reduced mRNA isoform diversity and neurodegeneration.
LaForce GR, Farr JS, Liu J, Akesson C, Gumus E, Pinkard O, Miranda HC, Johnson K, Sweet TJ, Ji P, Lin A, Coller J, Philippidou P, Wagner EJ, Schaffer AE
Neuron 2022 Apr 20;110(8):1340-1357.e7
Neuron 2022 Apr 20;110(8):1340-1357.e7
CPNE7-Induced Autophagy Restores the Physiological Function of Mature Odontoblasts.
Park YH, Son C, Seo YM, Lee YS, Har A, Park JC
Frontiers in cell and developmental biology 2021;9:655498
Frontiers in cell and developmental biology 2021;9:655498
Novel STMN2 Variant Linked to Amyotrophic Lateral Sclerosis Risk and Clinical Phenotype.
Theunissen F, Anderton RS, Mastaglia FL, Flynn LL, Winter SJ, James I, Bedlack R, Hodgetts S, Fletcher S, Wilton SD, Laing NG, MacShane M, Needham M, Saunders A, Mackay-Sim A, Melamed Z, Ravits J, Cleveland DW, Akkari PA
Frontiers in aging neuroscience 2021;13:658226
Frontiers in aging neuroscience 2021;13:658226
3D Visualization of Dynamic Cellular Reaction of Pulpal CD11c+ Dendritic Cells against Pulpitis in Whole Murine Tooth.
Hong S, Park YH, Lee J, Moon J, Kong E, Jeon J, Park JC, Kim HR, Kim P
International journal of molecular sciences 2021 Nov 24;22(23)
International journal of molecular sciences 2021 Nov 24;22(23)
Glycoproteomic analysis of the changes in protein N-glycosylation during neuronal differentiation in human-induced pluripotent stem cells and derived neuronal cells.
Kimura K, Koizumi T, Urasawa T, Ohta Y, Takakura D, Kawasaki N
Scientific reports 2021 May 27;11(1):11169
Scientific reports 2021 May 27;11(1):11169
SIRT1 regulates sphingolipid metabolism and neural differentiation of mouse embryonic stem cells through c-Myc-SMPDL3B.
Fan W, Tang S, Fan X, Fang Y, Xu X, Li L, Xu J, Li JL, Wang Z, Li X
eLife 2021 May 27;10
eLife 2021 May 27;10
microRNAs (miR 9, 124, 155 and 224) transdifferentiate mouse macrophages to neurons.
Challagundla N, Agrawal-Rajput R
Experimental cell research 2021 May 1;402(1):112563
Experimental cell research 2021 May 1;402(1):112563
Non-invasive and high-throughput interrogation of exon-specific isoform expression.
Truong DJ, Phlairaharn T, Eßwein B, Gruber C, Tümen D, Baligács E, Armbrust N, Vaccaro FL, Lederer EM, Beck EM, Geilenkeuser J, Göppert S, Krumwiede L, Grätz C, Raffl G, Schwarz D, Zirngibl M, Živanić M, Beyer M, Körner JD, Santl T, Evsyukov V, Strauß T, Schwarz SC, Höglinger GU, Heutink P, Doll S, Conrad M, Giesert F, Wurst W, Westmeyer GG
Nature cell biology 2021 Jun;23(6):652-663
Nature cell biology 2021 Jun;23(6):652-663
An epilepsy-associated ACTL6B variant captures neuronal hyperexcitability in a human induced pluripotent stem cell model.
Ahn LY, Coatti GC, Liu J, Gumus E, Schaffer AE, Miranda HC
Journal of neuroscience research 2021 Jan;99(1):110-123
Journal of neuroscience research 2021 Jan;99(1):110-123
Reproducible differentiation and characterization of neurons from mouse embryonic stem cells.
Saxena S, Choudhury S, Mohan KN
MethodsX 2020;7:101073
MethodsX 2020;7:101073
In vitro human stem cell derived cultures to monitor calcium signaling in neuronal development and function.
Sharma Y, Saha S, Joseph A, Krishnan H, Raghu P
Wellcome open research 2020;5:16
Wellcome open research 2020;5:16
A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids.
Renner H, Grabos M, Becker KJ, Kagermeier TE, Wu J, Otto M, Peischard S, Zeuschner D, TsyTsyura Y, Disse P, Klingauf J, Leidel SA, Seebohm G, Schöler HR, Bruder JM
eLife 2020 Nov 3;9
eLife 2020 Nov 3;9
Transcriptional signatures of participant-derived neural progenitor cells and neurons implicate altered Wnt signaling in Phelan-McDermid syndrome and autism.
Breen MS, Browne A, Hoffman GE, Stathopoulos S, Brennand K, Buxbaum JD, Drapeau E
Molecular autism 2020 Jun 19;11(1):53
Molecular autism 2020 Jun 19;11(1):53
Vimentin Coordinates Protein Turnover at the Aggresome during Neural Stem Cell Quiescence Exit.
Morrow CS, Porter TJ, Xu N, Arndt ZP, Ako-Asare K, Heo HJ, Thompson EAN, Moore DL
Cell stem cell 2020 Apr 2;26(4):558-568.e9
Cell stem cell 2020 Apr 2;26(4):558-568.e9
Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery.
Lingasamy P, Tobi A, Haugas M, Hunt H, Paiste P, Asser T, Rätsep T, Kotamraju VR, Bjerkvig R, Teesalu T
Biomaterials 2019 Oct;219:119373
Biomaterials 2019 Oct;219:119373
Comparison of in vitro Neuronal Differentiation Capacity Between Mouse Epiblast Stem Cells Derived From Nuclear Transfer and Naturally Fertilized Embryos.
Li T, Zheng Y, Li Y, Ye D
Frontiers in molecular neuroscience 2018;11:392
Frontiers in molecular neuroscience 2018;11:392
Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function.
Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, Tejada MA, Capilla-Gonzalez V, Rusanova I, Garcia-Verdugo JM, Acuña-Castroviejo D, López LC, Velez-Pardo C, Jimenez-Del-Rio M, Ferrer JM, Escames G
Journal of pineal research 2017 Sep;63(2)
Journal of pineal research 2017 Sep;63(2)
Comparative immunomorphology of testicular Sertoli and sertoliform tumors.
Mesa H, Gilles S, Datta MW, Murugan P, Larson W, Dachel S, Manivel JC
Human pathology 2017 Mar;61:181-189
Human pathology 2017 Mar;61:181-189
Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma.
Luo W, Li S, Peng B, Ye Y, Deng X, Yao K
PloS one 2013;8(2):e56324
PloS one 2013;8(2):e56324
Androgen receptors and gender-specific distribution of alkaline phosphatase in human thyroid cartilage.
Claassen H, Mönig H, Sel S, Werner JA, Paulsen F
Histochemistry and cell biology 2006 Sep;126(3):381-8
Histochemistry and cell biology 2006 Sep;126(3):381-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
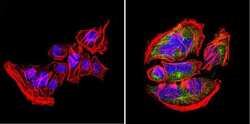
- Experimental details
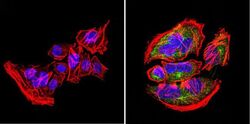
- Immunofluorescent analysis of nestin (green) in U251 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Monclonal Antibody (Product # MA1-110) at a dilution of 1:20 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
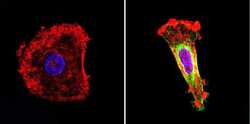
- Experimental details
- Immunofluorescent analysis of nestin (green) in human cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Monclonal Antibody (Product # MA1-110) at a dilution of 1:20 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
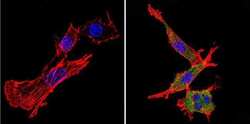
- Experimental details
- Immunofluorescent analysis of nestin (green) in murine cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Monclonal Antibody (Product # MA1-110) at a dilution of 1:20 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
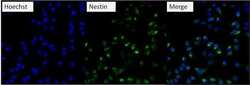
- Experimental details
- Immunofluorescent analysis of Nestin (green) U2-0S cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 0.3% BSA/TBST (Product # 37525) for 15 minutes at room temperature. Cells were probed with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1:200 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat-anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:400 for 30 minutes at room temperature. Nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
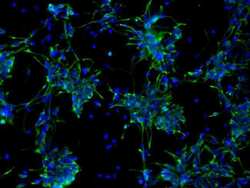
- Experimental details
- Immunofluorescent analysis of Nestin (green) in human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). The cells were fixed and permeabilized using Image-IT® Fixation/Permeabilization kit (Product # R37602), and blocked with the including blocking buffer for one hour at room temperature. Cells were stained with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1:100 in blocking buffer for 3 hours at room temperature, and then incubated with a DyLight488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:250 for 1 hour at room temperature. Nucleus DNA (blue) was stained with DAPI (Product # D1306). Images were taken on an EVOS® FLoid® Cell Imaging Station at 10X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
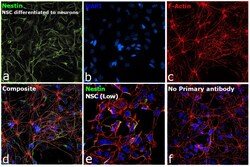
- Experimental details
- Immunofluorescence analysis of Nestin was performed using 70% confluent log phase NSC differentiated to neurons. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Nestin Monoclonal Antibody (10C2) (Product # MA1-110) at 1:250 in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32766), (1:2500), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Cytosolic localization. Panel e represents NSC with lower expression of Nestin. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
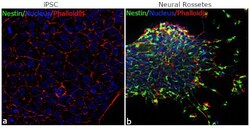
- Experimental details
- For immunofluorescence analysis, iPSC differentiated to Neural Rosettes were fixed and permeabilized for detection of endogenous Nestin using Anti-Nestin mouse monoclonal antibody (Product # MA1-110, 1:100 dilution) and labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27023, 1:2000). Panel b) shows representative cells that were stained for detection and localization of Nestin protein (green) in the cytoplasm of Neural Rosettes in comparison to iPSC (a).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of nestin (green) in U251 cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Monclonal Antibody (Product # MA1-110) at a dilution of 1:20 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
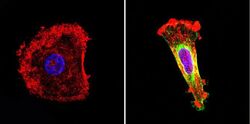
- Experimental details
- Immunofluorescent analysis of nestin (green) in human cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Monclonal Antibody (Product # MA1-110) at a dilution of 1:20 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
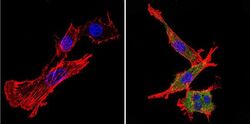
- Experimental details
- Immunofluorescent analysis of nestin (green) in murine cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Monclonal Antibody (Product # MA1-110) at a dilution of 1:20 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
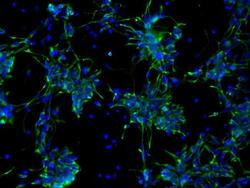
- Experimental details
- Immunofluorescent analysis of Nestin (green) in human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). The cells were fixed and permeabilized using Image-IT® Fixation/Permeabilization kit (Product # R37602), and blocked with the including blocking buffer for one hour at room temperature. Cells were stained with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1:100 in blocking buffer for 3 hours at room temperature, and then incubated with a DyLight488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:250 for 1 hour at room temperature. Nucleus DNA (blue) was stained with DAPI (Product # D1306). Images were taken on an EVOS® FLoid® Cell Imaging Station at 10X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
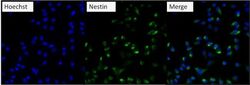
- Experimental details
- Immunofluorescent analysis of Nestin (green) U2-0S cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 0.3% BSA/TBST (Product # 37525) for 15 minutes at room temperature. Cells were probed with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1:200 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat-anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:400 for 30 minutes at room temperature. Nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
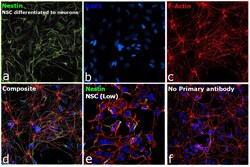
- Experimental details
- Immunofluorescence analysis of Nestin was performed using 70% confluent log phase NSC differentiated to neurons. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with Nestin Monoclonal Antibody (10C2) (Product # MA1-110) at 1:250 in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32766), (1:2500), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Cytosolic localization. Panel e represents NSC with lower expression of Nestin. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
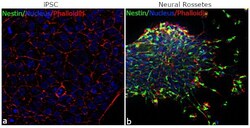
- Experimental details
- For immunofluorescence analysis, iPSC differentiated to Neural Rosettes were fixed and permeabilized for detection of endogenous Nestin using Anti-Nestin mouse monoclonal antibody (Product # MA1-110, 1:100 dilution) and labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27023, 1:2000). Panel b) shows representative cells that were stained for detection and localization of Nestin protein (green) in the cytoplasm of Neural Rosettes in comparison to iPSC (a).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
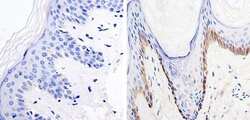
- Experimental details
- Immunohistochemistry was performed on human skin tissue. To expose target protein, antigen was retreived using 10mM sodium citrate followed by microwave treatment for 8-15 minutes. Endogenous peroxidases were blocked in 3% H202-methanol for 15 minutes and tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Rabbit polyclonal antibody (Product # MA1-110) at a dilution of 1:20 overnight in a humidified chamber. Tissues were washed in PBST and detection was performed using a secondary antibody conjugated to HRP. DAB staining buffer was applied and tissues were counterstained with hematoxylin and prepped for mounting. Images were taken at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
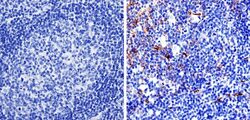
- Experimental details
- Immunohistochemistry was performed on human tonsil tissue. To expose target protein, antigen was retreived using 10mM sodium citrate followed by microwave treatment for 8-15 minutes. Endogenous peroxidases were blocked in 3% H202-methanol for 15 minutes and tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Rabbit polyclonal antibody (Product # MA1-110) at a dilution of 1:100 overnight in a humidified chamber. Tissues were washed in PBST and detection was performed using a secondary antibody conjugated to HRP. DAB staining buffer was applied and tissues were counterstained with hematoxylin and prepped for mounting. Images were taken at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
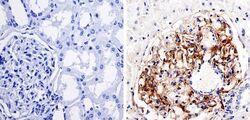
- Experimental details
- Immunohistochemistry was performed on human kidney tissue. To expose target protein, antigen was retreived using 10mM sodium citrate followed by microwave treatment for 8-15 minutes. Endogenous peroxidases were blocked in 3% H202-methanol for 15 minutes and tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a nestin Rabbit polyclonal antibody (Product # MA1-110) at a dilution of 1:100 overnight in a humidified chamber. Tissues were washed in PBST and detection was performed using a secondary antibody conjugated to HRP. DAB staining buffer was applied and tissues were counterstained with hematoxylin and prepped for mounting. Images were taken at 40X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
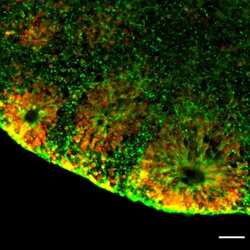
- Experimental details
- Immunofluorescent analysis of NESTIN (green) and SOX2 (red) in human iPSC-derived forebrain organoids derived at Day 40. The organoids were fixed with 4% PFA for 1 hour at room temperature, followed by incubation with 30% sucrose solution overnight at 4°C. The organoids were then embedded in OCT and cryosectioned at 5 µm, permeabilized with 0.2% Triton X-100 for 20 min, and blocked with 10% donkey serum in PBS for 30 min at room temperature. Organoid slices were stained with a Mouse NESTIN monoclonal antibody (green; Product # MA1-110) at a dilution of 1:500, and a Rabbit SOX2 polyclonal antibody (red; Product # PA1-094X) at a dilution of 1:500 in blocking buffer overnight at 4°C, and then incubated with Donkey anti-Mouse Alexa Fluor 488 (Product # R37114) and Donkey anti-Rabbit Alexa Fluor 568 (Product # A10042) at a dilution of 1:1000 in blocking solution at room temperature for 1 hour. Images were taken at 20X magnification. Scale bar: 50 µm. Data courtesy of Dr. Zhexing Wen at Emory University.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
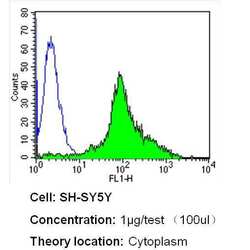
- Experimental details
- Flow cytometry analysis of Nestin in SH-SY5Y cells (green) compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1 µg/test for 40 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
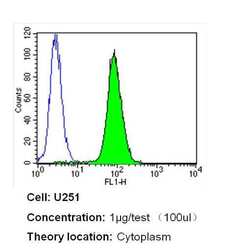
- Experimental details
- Flow cytometry analysis of Nestin in U251 cells (green) compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1 µg/test for 40 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
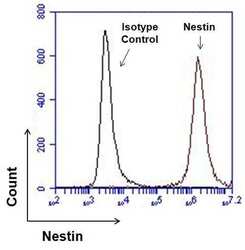
- Experimental details
- Flow cytometry analysis of Nestin on human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). Cells were fixed, permeabilized, and then stained with 1 µg of Nestin monoclonal antibody (Product # MA1-110, red histogram) or mouse IgG1 isotype control (black histogram) in 5% BSA. After incubation of the primary antibody for 1 hour on ice, the cells were stained with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:500 for 1 hour on ice. A representative 10,000 cells were acquired for each sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
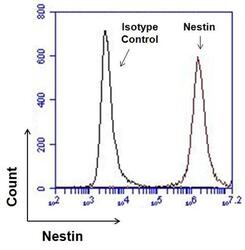
- Experimental details
- Flow cytometry analysis of Nestin on human neural stem cells derived from PD-3 iPSCs using Gibco® PSC Neural Induction Medium (Product # A1647801). Cells were fixed, permeabilized, and then stained with 1 µg of Nestin monoclonal antibody (Product # MA1-110, red histogram) or mouse IgG1 isotype control (black histogram) in 5% BSA. After incubation of the primary antibody for 1 hour on ice, the cells were stained with a DyLight 488-conjugated goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:500 for 1 hour on ice. A representative 10,000 cells were acquired for each sample.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
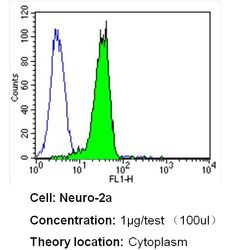
- Experimental details
- Flow cytometry analysis of Nestin in Neuro-2a cells (green) compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1 µg/test for 40 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
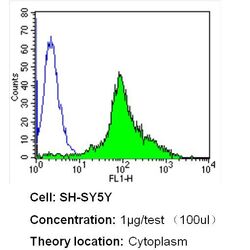
- Experimental details
- Flow cytometry analysis of Nestin in SH-SY5Y cells (green) compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1 µg/test for 40 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
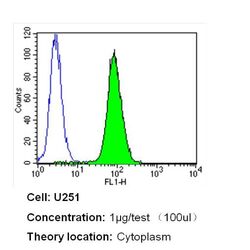
- Experimental details
- Flow cytometry analysis of Nestin in U251 cells (green) compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Nestin monoclonal antibody (Product # MA1-110) at a dilution of 1 µg/test for 40 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated secondary antibody and re-suspended in PBS for FACS analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
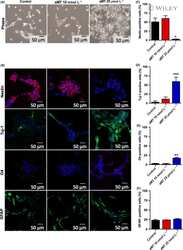
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
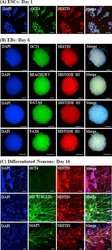
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
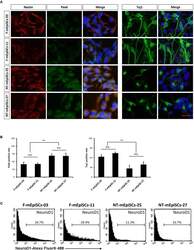
- Experimental details
- FIGURE 4 Quantification of early neurons derived from F-mEpiSCs and NT-mEpiSCs. (A) Immunofluorescence staining for Nestin, Pax6, and Tuj1 of early neurons derived from F-mEpiSCs and NT-mEpiSCs; nuclei are shown using DAPI (B) Quantification of Tuj1-positive and Pax6-positive cells based on Tuj1 and Pax6 immunofluorescence staining. (C) Flow cytometry analysis of F-EpiSCs and NT-EpiSCs stained for the neuronal marker NeuroD1. Mouse Neuro-2A cells served as positive control for the staining. Data were acquired for a minimum of 10 x 10 3 events per sample using the FC500 flow cytometer and analyzed using the FlowJo software. Scale bar: 25 mum; n.s., P > 0.05; ** P < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
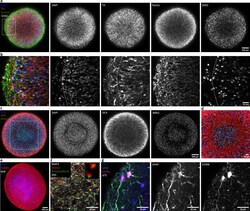
- Figure 2. Automated midbrain organoids express typical neural and midbrain markers and show signs of structural organization. ( a ) Expression of the dopaminergic midbrain marker TH as well as the precursor markers nestin and Sox2 is evenly distributed throughout the entire aggregate at day 25, as shown by single confocal microscopy slices of tissue-cleared samples. The dotted box indicates the area shown in ( b ). Here, higher magnification of the peripheral aggregate region reveals two different zones with few nuclei but dense, circumferentially oriented neurites distally from the core and radial organization of TH-positive neurons more proximally. ( c ) The expression patterns of DCX and Brn2 further illustrate the organization of neurons (DCX) and neural precursors (Brn2) in the core of AMOs into zones. ( d ) Enlargement (of the dotted box in c) highlighting the circumferential organization of neurons (DCX) surrounding the core. ( e ) Maximum intensity projection (MIP) of fluorescent confocal images showing a dense cellular network expressing the neural marker beta-tubulin III (TUBB33) within the AMOs at d25. ( f/g ) Continuing maturation of AMOs is indicated by the presence of synapses marked by the colocalization of the presynaptic synaptophysin and postsynaptic homer on Map2-positive neurites at day 50 (f, top right corner showing enlargement of two synapses without the Map2 channel) and S100b/GFAP double-positive astrocytes at day 75 ( g ). Scale bars: 100 mum ( a, c,
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
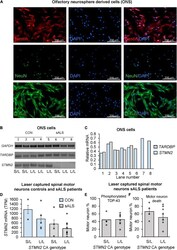
- FIGURE 3 The effect of CA genotype on STMN2 mRNA expression in olfactory neurosphere derived cell lines and laser captured spinal motor neurons. (A) Immunostaining of ONS cells with Nestin, NeuN, and beta-tubulin. (B) Variable expression of STMN2 in sALS ONS cell lines (lanes 5-8) compared to control ONS cell lines (lanes 1-4). (C) Relative densitometry of TARDBP and STMN2 standardized to GAPDH expression showing variable expression of STMN2 in sALS cell lines and no difference in TARDBP . (D) Trend for a stepwise decrease in STMN2 mRNA expression between control and sALS cases according to STMN2 CA genotype (S/L vs. L/L), error bars represent standard error of the mean. RNA sequencing data was analyzed by and STMN2 expression between cases and controls previously reported (). (E) Percentage of motor neurons positive for phosphorylated TDP-43 during initial sample collection done by , according to STMN2 CA genotype. (F) Percentage of motor neuron death according to STMN2 CA genotype based on data collected by .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
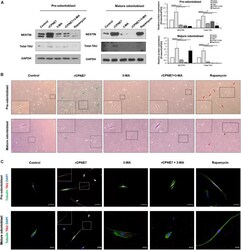
- FIGURE 3 CPNE7 stimulates odontoblast process elongation. (A) Both stages of hDPCs were treated with rCPNE7, 3-MA, rCPNE7 + 3-MA, or rapamycin for 24 h. NESTIN and total-TAU protein levels were evaluated by western blot analysis. (B,C) The morphology of hDPCs was analyzed in each group 7 days after treatment. Boxed areas are shown at higher magnification. (B) Cells were observed under an optical microscope (100 x). Odontoblasts with a unidirectional odontoblast process-like structure (green arrows) were observed in the rCPNE7 group. Multidirectional odontoblast process-like structures were observed (red arrowheads) in the rapamycin group. (C) Representative immunofluorescence images of TUBULIN (green) and TAU (red) in each group of treated cells. TUBULIN and TAU expression was co-localized in the odontoblast process-like structures in the rCPNE7 group (arrows). Scale bars = 50 mum and 20 mum. Significant differences are shown with asterisks. * P < 0.05.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
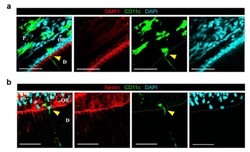
- Figure 2 CD11c+ cell at dentin-pulp interface does not express odontoblast differentiation markers of DMP1 and Nestin. ( a ) Single-plane images of the tooth extracted from CD11c-YFP mouse after immunostaining of DMP1. Yellow arrowhead marks CD11c+ cell at the dentin-pulp interface. Scale bar, 50 mum. DMP1 (red), CD11c (green), DAPI (cyan). ( b ) Single plane images of the tooth extracted from CD11c-YFP mouse after immunostaining of Nestin. Yellow arrowhead marks CD11c+ cell at the dentin-pulp interface. Scale bar, 50 mum. Nestin (red), CD11c (green), DAPI (cyan). White dotted lines delineate the dentin-pulp interface. D : dentin, P : pulp, OB : odontoblast layer.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
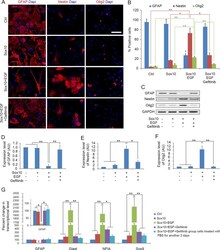
- Experimental details
- Fig. 2 EGF promotes astrocyte dedifferentiation. A Immunostaining of cells with GFAP, Nestin and Olig2 antibodies 5 days after transdifferentiation. B Quantification of immune-positive cells in the four cultures. C Western blotting analysis of GFAP, Nestin and Olig2 on d5 during the transdifferentiation process. D - F Quantitative analysis of GFAP, Nestin and Olig2 proteins expression in the four cultures on d5, respectively, histograms express results in arbitrary units, taking Ctrl cells values as 100%. G Quantitative RT-PCR analysis of the expression level of GFAP, Glast, NFIA and Sox9 in the four groups on d5 during the transdifferentiation process and Sox10 + EGF + Gefitinib group cells treated with FBS for another 3 days. Statistical analyses are presented as Mean +- SD, n = 3. **P < 0.01, ***P < 0.001. Scale bars, 50 mum
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
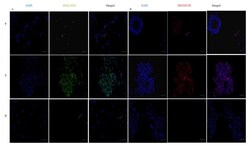
- Experimental details
- Expression of stemness markers: immunocytochemistry images of ( A ) Sox2 primary antibody stained with FITC and counterstained with DAPI; ( B ) Nestin-primary antibody stained with PE and counterstained with DAPI. The scale bar indicates 20 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
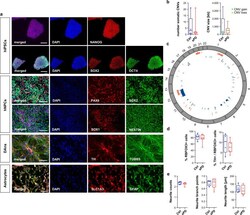
- Experimental details
- Characterization of hiPSC derived hNPCs, neurons and astrocytes. a Immunostainings exemplarily shown for O3H-R1-003. hiPSC pluripotency staining for markers OCT4, NANOG, SOX2. Scale bar=200 um. hNPC staining for markers SOX1, SOX2, NESTIN, PAX6. Scale bar=100 um. Neuron staining for markers TUBB3 and DAn marker TH. Scale bar=100 um. Astrocyte staining for markers GFAP and SLC1A3. Scale bar=100 um. b Summary of somatic CNVs identified in hNPC clones by chromosomal microarray analysis shown as total number of somatic CNVs detected per analyzed clone and average length of CNVs (in kb; green=copy number gain; orange=copy number loss) per analyzed clone. n = 5 Ctrl and 7 sPD patients. c Circos plot showing the genomic distribution of somatic CNVs in Ctrl (blue) and sPD (red) clones. d Quantification of RBFOX3 (synonym: NeuN) positive as well as TH / RBFOX3 double-positive cells in DAn populations. n = 5 Ctrl and 7 sPD clones, in triplicates. e Characterization of neurite morphologies of DAns. Boxplots show the average number of neurites emerging from TH positive cell bodies, their average number of branch points and their average length. n = 5 Ctrl and 7 sPD clones, in triplicates. Boxplots display the median and range from the 25th to 75th percentile. Whiskers extend from the min to max value. Each dot represents one patient. P -values were determined by two-sided t -test d (right), e ; two-sided Mann-Whitney-U test b, d (left). * p < 0.05, ** p < 0.01, *** p < 0.001. Source data
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
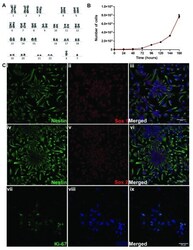
- Experimental details
- Figure 1. Characterization of XCL-1 neural stem cells (NSCs). ( A ) Cytogenetic characterization of NSCs by karyotyping. Image of chromosome from a metaphase spread of XCL1 showing normal number and banding pattern of each of the 22 pairs of autosomes, X and Y chromosomes. ( B ) Proliferation curve of XCL1, with the values represented as mean +- SEM of 3 independent measurements. Y-axis depicts the number of cells and the X-axis, age in hours ( C ) Confocal images of XCL-1 showing the expression of (i-iii) Nestin/SOX1. Confocal images of (iv-vi) Nestin/SOX2 and (vii-ix) Ki-67/DAPI. The NSCs were regularly observed in their typical neural rosette morphology. The confocal images in this panel were brightness adjusted using ImageJ for better visualization. Scale bar 50 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
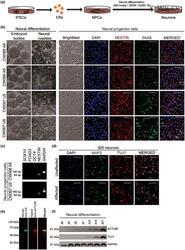
- Experimental details
- 3 FIGURE Neural differentiation of patient-derived induced pluripotent stem cells (iPSCs) to neural progenitor cells and neurons. (a) Schematic of neural differentiation from EIEE-76 patient-specific iPSCs. (b) Neural progenitor cells (NPCs) differentiated from control (Unaffected: CW067 U5; CW067 U9) and an EIEE-76 patient (Affected: CW066 A4; CW066 A5) iPSC clones are indistinguishable. The iPSCs clones form embryoid bodies (EBs) and neural rosettes (yellow, dashed outline) similarly upon neural induction. Isolated NPCs express neural progenitor cell markers PAX6 and NESTIN. Representative images shown with DAPI (4',6-diamidino-2-phenylindole) counterstain. Scale bar, 100 mum. (c) Characterization of NPC lines derived from unaffected familial control (CW067) and an EIEE-76 affected patient (CW066) by RT-PCR for expression of neural crest cell ( SOX10 and FOXD3), stem cell ( OCT4), and neural progenitor cell ( NESTIN ) identity genes show the NPC cultures are not contaminated by neural crest derivatives or undifferentiated stem cells. GAPDH , loading control. (d) Co-immunocytochemistry for neuronal markers MAP2 and TUJ1 at day (d)25 of differentiation show positive expression in familial unaffected controls and EIEE-76 patient-specific affected neurons. Representative images shown with DAPI counterstain. Scale bar, 50 mum. (e) Dual color infrared western blot for HA (green) and ACTL6B (red) in HEK293T cell lysates transfected with a 2xHA-epitope tagged ACTL6B expression cons
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry