Antibody data
- Antibody Data
- Antigen structure
- References [141]
- Comments [0]
- Validations
- Immunocytochemistry [3]
- Immunohistochemistry [3]
- Other assay [67]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-913 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Calsequestrin Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Purifed from natural sources
- Description
- PA1-913 detects calsequestrin from canine, human, mouse, rat and sheep tissues. This antibody recognizes both cardiac and skeletal muscle calsequestrin. PA1-913 has been successfully used in Western blot, immunofluorescence, immunocytochemistry, immunohistochemistry (paraffin) and immunoprecipitation procedures. By Western blot, this antibody detects an ~55 kDa protein representing calsequestrin from canine cardiac extract. Additional bands at 97 kDa may be observed and have been reported to be calsequestrin-like proteins. PA1-913 antigen is purified canine cardiac calsequestrin.
- Reactivity
- Human, Mouse, Rat, Canine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Anti-inflammatory effects of endothelin receptor blockade in left atrial tissue of spontaneously hypertensive rats.
Phosphorylation at Serines 157 and 161 Is Necessary for Preserving Cardiac Expression Level and Functions of Sarcomeric Z-Disc Protein Telethonin.
Parvalbumin affects skeletal muscle trophism through modulation of mitochondrial calcium uptake.
Sarcolipin haploinsufficiency prevents dystrophic cardiomyopathy in mdx mice.
Calsequestrins New Calcium Store Markers of Adult Zebrafish Cerebellum and Optic Tectum.
Lack of functional wolframin causes drop in plasmalemmal sodium-calcium exchanger type 1 expression at early stage in rat model of Wolfram syndrome.
Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice.
Calsequestrin Deletion Facilitates Hippocampal Synaptic Plasticity and Spatial Learning in Post-Natal Development.
Inward Rectifier K(+) Currents Contribute to the Proarrhythmic Electrical Phenotype of Atria Overexpressing Cyclic Adenosine Monophosphate Response Element Modulator Isoform CREM-IbΔC-X.
Phosphorylation of cardiac myosin-binding protein-C contributes to calcium homeostasis.
Altered calcium handling produces reentry-promoting action potential alternans in atrial fibrillation-remodeled hearts.
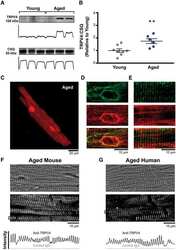
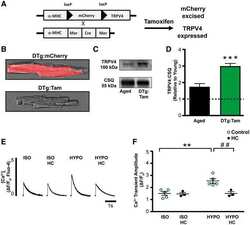
TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress.
Plakophilin-2 Haploinsufficiency Causes Calcium Handling Deficits and Modulates the Cardiac Response Towards Stress.
Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients With and Without Atrial Fibrillation.
Regulation of BECN1-mediated autophagy by HSPB6: Insights from a human HSPB6(S10F) mutant.
The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy.
Facilitation of ischaemia-induced ventricular fibrillation by catecholamines is mediated by β(1) and β(2) agonism in the rat heart in vitro.
Conditional ablation and conditional rescue models for Casq2 elucidate the role of development and of cell-type specific expression of Casq2 in the CPVT2 phenotype.
Inflammation leads through PGE/EP(3) signaling to HDAC5/MEF2-dependent transcription in cardiac myocytes.
A secretory pathway kinase regulates sarcoplasmic reticulum Ca(2+) homeostasis and protects against heart failure.
A chemical chaperone improves muscle function in mice with a RyR1 mutation.
Calcium dysregulation, functional calpainopathy, and endoplasmic reticulum stress in sporadic inclusion body myositis.
T-tubule remodelling disturbs localized β2-adrenergic signalling in rat ventricular myocytes during the progression of heart failure.
The TRPM4 channel is functionally important for the beneficial cardiac remodeling induced by endurance training.
Gene-Targeted Mice with the Human Troponin T R141W Mutation Develop Dilated Cardiomyopathy with Calcium Desensitization.
Genotype-Dependent and -Independent Calcium Signaling Dysregulation in Human Hypertrophic Cardiomyopathy.
Acyl CoA synthetase-1 links facilitated long chain fatty acid uptake to intracellular metabolic trafficking differently in hearts of male versus female mice.
The angiotensin receptor-associated protein Atrap is a stimulator of the cardiac Ca2+-ATPase SERCA2a.
Heterozygous deletion of sarcolipin maintains normal cardiac function.
Protein Phosphatase 1c Associated with the Cardiac Sodium Calcium Exchanger 1 Regulates Its Activity by Dephosphorylating Serine 68-phosphorylated Phospholemman.
The maintenance ability and Ca(2+) availability of skeletal muscle are enhanced by sildenafil.
Cannabinoid signalling inhibits sarcoplasmic Ca(2+) release and regulates excitation-contraction coupling in mammalian skeletal muscle.
Development of a high-affinity peptide that prevents phospholemman (PLM) inhibition of the sodium/calcium exchanger 1 (NCX1).
Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding.
Ca(2+) permeation and/or binding to CaV1.1 fine-tunes skeletal muscle Ca(2+) signaling to sustain muscle function.
Effectiveness of gene delivery systems for pluripotent and differentiated cells.
The Effect of SERCA1b Silencing on the Differentiation and Calcium Homeostasis of C2C12 Skeletal Muscle Cells.
Suppression of Early and Late Afterdepolarizations by Heterozygous Knockout of the Na+/Ca2+ Exchanger in a Murine Model.
Expression of calcium-buffering proteins in rat intrinsic laryngeal muscles.
Sox9 expression in canine epithelial skin tumors.
Metabolic efficiency promotes protection from pressure overload in hearts expressing slow skeletal troponin I.
Changes in the cardiac metabolome caused by perhexiline treatment in a mouse model of hypertrophic cardiomyopathy.
p11 modulates calcium handling through 5-HT₄R pathway in rat ventricular cardiomyocytes.
Mitsugumin 56 (hedgehog acyltransferase-like) is a sarcoplasmic reticulum-resident protein essential for postnatal muscle maturation.
Regional ion channel gene expression heterogeneity and ventricular fibrillation dynamics in human hearts.
Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age.
Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation.
The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias.
Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release.
Molecular basis of calpain cleavage and inactivation of the sodium-calcium exchanger 1 in heart failure.
The PEG-switch assay: a fast semi-quantitative method to determine protein reversible cysteine oxidation.
Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart.
Noncanonical EF-hand motif strategically delays Ca2+ buffering to enhance cardiac performance.
The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy.
Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy.
Comparative differential proteomic profiles of nonfailing and failing hearts after in vivo thoracic aortic constriction in mice overexpressing FKBP12.6.
Knockout of the Na,K-ATPase α2-isoform in cardiac myocytes delays pressure overload-induced cardiac dysfunction.
Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy.
Functional scaffold-free 3-D cardiac microtissues: a novel model for the investigation of heart cells.
CREB critically regulates action potential shape and duration in the adult mouse ventricle.
Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes.
Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias.
Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction.
Functional evidence for an active role of B-type natriuretic peptide in cardiac remodelling and pro-arrhythmogenicity.
Role of Junctin protein interactions in cellular dynamics of calsequestrin polymer upon calcium perturbation.
Immortalization of bone marrow-derived porcine mesenchymal stem cells and their differentiation into cells expressing cardiac phenotypic markers.
4D super-resolution microscopy with conventional fluorophores and single wavelength excitation in optically thick cells and tissues.
Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway.
Differential effect of calsequestrin ablation on structure and function of fast and slow skeletal muscle fibers.
Organic cation transporter 3: expression in failing and nonfailing human heart and functional characterization.
Catecholaminergic-induced arrhythmias in failing cardiomyocytes associated with human HRCS96A variant overexpression.
Knockout of the Na,K-ATPase α₂-isoform in the cardiovascular system does not alter basal blood pressure but prevents ACTH-induced hypertension.
FKBP12.6 mice display temporal gender differences in cardiac Ca(2+)-signalling phenotype upon chronic pressure overload.
Antioxidant network expression abrogates oxidative posttranslational modifications in mice.
Prevention of ventricular arrhythmia and calcium dysregulation in a catecholaminergic polymorphic ventricular tachycardia mouse model carrying calsequestrin-2 mutation.
Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function.
Conserved expression and functions of PDE4 in rodent and human heart.
Impaired β-adrenergic responsiveness accentuates dysfunctional excitation-contraction coupling in an ovine model of tachypacing-induced heart failure.
SR-targeted CaMKII inhibition improves SR Ca²+ handling, but accelerates cardiac remodeling in mice overexpressing CaMKIIδC.
STAT subtype specificity and ischemic preconditioning in mice: is STAT-3 enough?
Couplons in rat atria form distinct subgroups defined by their molecular partners.
Caspase-dependent protein phosphatase 2A activation contributes to endotoxin-induced cardiomyocyte contractile dysfunction.
Mitochondrial uncoupling downregulates calsequestrin expression and reduces SR Ca2+ stores in cardiomyocytes.
Superior calcium homeostasis of extraocular muscles.
Sequential alterations in Akt, GSK3β, and calcineurin signalling in the mouse left ventricle after thoracic aortic constriction.
S165F mutation of junctophilin 2 affects Ca2+ signalling in skeletal muscle.
Carbon monoxide pollution promotes cardiac remodeling and ventricular arrhythmia in healthy rats.
Chronic heart rate reduction with ivabradine improves systolic function of the reperfused heart through a dual mechanism involving a direct mechanical effect and a long-term increase in FKBP12/12.6 expression.
A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling.
Postnatal development of mouse heart: formation of energetic microdomains.
The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering.
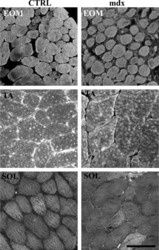
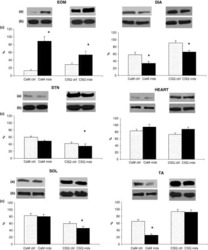
Calcium-binding proteins in skeletal muscles of the mdx mice: potential role in the pathogenesis of Duchenne muscular dystrophy.
Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice.
Diabetes-related defects in sarcoplasmic Ca2+ release are prevented by inactivation of G(alpha)11 and G(alpha)q in murine cardiomyocytes.
Effects of AT1- and beta-adrenergic receptor antagonists on TGF-beta1-induced fibrosis in transgenic mice.
Altered contractility of skeletal muscle in mice deficient in titin's M-band region.
Diltiazem and verapamil protect dystrophin-deficient muscle fibers of MDX mice from degeneration: a potential role in calcium buffering and sarcolemmal stability.
Reduced expression of sarcalumenin and related Ca2+ -regulatory proteins in aged rat skeletal muscle.
Single histidine-substituted cardiac troponin I confers protection from age-related systolic and diastolic dysfunction.
Cardiac-directed parvalbumin transgene expression in mice shows marked heart rate dependence of delayed Ca2+ buffering action.
Enhanced calcium cycling and contractile function in transgenic hearts expressing constitutively active G alpha o* protein.
S100A1 deficiency results in prolonged ventricular repolarization in response to sympathetic activation.
Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo.
Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model.
Interstitial remodeling in beta1-adrenergic receptor transgenic mice.
Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes.
Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia.
High intracellular Na+ preserves myocardial function at low heart rates in isolated myocardium from failing hearts.
Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death.
LAMP-2 deficient mice show depressed cardiac contractile function without significant changes in calcium handling.
Association of RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy.
Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction.
Increased susceptibility to isoproterenol-induced cardiac hypertrophy and impaired weight gain in mice lacking the histidine-rich calcium-binding protein.
Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure.
Myotonic dystrophy protein kinase phosphorylates phospholamban and regulates calcium uptake in cardiomyocyte sarcoplasmic reticulum.
Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies.
Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes.
Phosphorylation-status of phospholamban and calsequestrin modifies their affinity towards commonly used antibodies.
Regulation of dihydropyridine receptor gene expression in mouse skeletal muscles by stretch and disuse.
The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm.
Human homozygous R403W mutant cardiac myosin presents disproportionate enhancement of mechanical and enzymatic properties.
Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity.
Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin.
Phospholamban gene ablation improves calcium transients but not cardiac function in a heart failure model.
Effects of chronic endothelin-1 stimulation on cardiac myocyte contractile function.
Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia.
Na(+)-Ca(2+) exchanger overexpression predisposes to reactive oxygen species-induced injury.
Na(+)-Ca(2+) exchanger overexpression predisposes to reactive oxygen species-induced injury.
Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17.
Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol.
Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat.
Supramolecular calsequestrin complex.
Supramolecular calsequestrin complex.
Augmented expression of cardiotrophin-1 in failing human hearts is accompanied by diminished glycoprotein 130 receptor protein abundance.
A novel and rapid approach to isolating functional ryanodine receptors.
Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality.
Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality.
Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium.
Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane.
Residues 2-25 of phospholamban are insufficient to inhibit Ca2+ transport ATPase of cardiac sarcoplasmic reticulum.
Developmental changes in cardiac sarcoplasmic reticulum in sheep.
Bukowska A, Nikonova Y, Wolke C, Lendeckel U, Kockskämper J, Goette A
International journal of cardiology. Heart & vasculature 2022 Oct;42:101088
International journal of cardiology. Heart & vasculature 2022 Oct;42:101088
Phosphorylation at Serines 157 and 161 Is Necessary for Preserving Cardiac Expression Level and Functions of Sarcomeric Z-Disc Protein Telethonin.
Lewis HR, Eminaga S, Gautel M, Avkiran M
Frontiers in physiology 2021;12:732020
Frontiers in physiology 2021;12:732020
Parvalbumin affects skeletal muscle trophism through modulation of mitochondrial calcium uptake.
Butera G, Vecellio Reane D, Canato M, Pietrangelo L, Boncompagni S, Protasi F, Rizzuto R, Reggiani C, Raffaello A
Cell reports 2021 May 4;35(5):109087
Cell reports 2021 May 4;35(5):109087
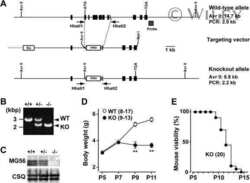
Sarcolipin haploinsufficiency prevents dystrophic cardiomyopathy in mdx mice.
Mareedu S, Pachon R, Thilagavathi J, Fefelova N, Balakrishnan R, Niranjan N, Xie LH, Babu GJ
American journal of physiology. Heart and circulatory physiology 2021 Jan 1;320(1):H200-H210
American journal of physiology. Heart and circulatory physiology 2021 Jan 1;320(1):H200-H210
Calsequestrins New Calcium Store Markers of Adult Zebrafish Cerebellum and Optic Tectum.
Furlan S, Campione M, Murgia M, Mosole S, Argenton F, Volpe P, Nori A
Frontiers in neuroanatomy 2020;14:15
Frontiers in neuroanatomy 2020;14:15
Lack of functional wolframin causes drop in plasmalemmal sodium-calcium exchanger type 1 expression at early stage in rat model of Wolfram syndrome.
Kureková S, Plaas M, Cagalinec M
General physiology and biophysics 2020 Sep;39(5):499-503
General physiology and biophysics 2020 Sep;39(5):499-503
Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice.
Parahuleva MS, Kockskämper J, Heger J, Grimm W, Scherer A, Bühler S, Kreutz J, Schulz R, Euler G
International journal of molecular sciences 2020 Nov 30;21(23)
International journal of molecular sciences 2020 Nov 30;21(23)
Calsequestrin Deletion Facilitates Hippocampal Synaptic Plasticity and Spatial Learning in Post-Natal Development.
Ambrogini P, Lattanzi D, Di Palma M, Ciacci C, Savelli D, Galati C, Gioacchini AM, Pietrangelo L, Vallorani L, Protasi F, Cuppini R
International journal of molecular sciences 2020 Jul 31;21(15)
International journal of molecular sciences 2020 Jul 31;21(15)
Inward Rectifier K(+) Currents Contribute to the Proarrhythmic Electrical Phenotype of Atria Overexpressing Cyclic Adenosine Monophosphate Response Element Modulator Isoform CREM-IbΔC-X.
Pluteanu F, Seidl MD, Hamer S, Scholz B, Müller FU
Journal of the American Heart Association 2020 Dec;9(23):e016144
Journal of the American Heart Association 2020 Dec;9(23):e016144
Phosphorylation of cardiac myosin-binding protein-C contributes to calcium homeostasis.
Kumar M, Haghighi K, Kranias EG, Sadayappan S
The Journal of biological chemistry 2020 Aug 7;295(32):11275-11291
The Journal of biological chemistry 2020 Aug 7;295(32):11275-11291
Altered calcium handling produces reentry-promoting action potential alternans in atrial fibrillation-remodeled hearts.
Liu T, Xiong F, Qi XY, Xiao J, Villeneuve L, Abu-Taha I, Dobrev D, Huang C, Nattel S
JCI insight 2020 Apr 7;5(8)
JCI insight 2020 Apr 7;5(8)
TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress.
Jones JL, Peana D, Veteto AB, Lambert MD, Nourian Z, Karasseva NG, Hill MA, Lindman BR, Baines CP, Krenz M, Domeier TL
Cardiovascular research 2019 Jan 1;115(1):46-56
Cardiovascular research 2019 Jan 1;115(1):46-56
Plakophilin-2 Haploinsufficiency Causes Calcium Handling Deficits and Modulates the Cardiac Response Towards Stress.
van Opbergen CJM, Noorman M, Pfenniger A, Copier JS, Vermij SH, Li Z, van der Nagel R, Zhang M, de Bakker JMT, Glass AM, Mohler PJ, Taffet SM, Vos MA, van Rijen HVM, Delmar M, van Veen TAB
International journal of molecular sciences 2019 Aug 21;20(17)
International journal of molecular sciences 2019 Aug 21;20(17)
Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients With and Without Atrial Fibrillation.
Molina CE, Abu-Taha IH, Wang Q, Roselló-Díez E, Kamler M, Nattel S, Ravens U, Wehrens XHT, Hove-Madsen L, Heijman J, Dobrev D
Frontiers in physiology 2018;9:1383
Frontiers in physiology 2018;9:1383
Regulation of BECN1-mediated autophagy by HSPB6: Insights from a human HSPB6(S10F) mutant.
Liu GS, Zhu H, Cai WF, Wang X, Jiang M, Essandoh K, Vafiadaki E, Haghighi K, Lam CK, Gardner G, Adly G, Nicolaou P, Sanoudou D, Liang Q, Rubinstein J, Fan GC, Kranias EG
Autophagy 2018;14(1):80-97
Autophagy 2018;14(1):80-97
The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy.
Makarewich CA, Munir AZ, Schiattarella GG, Bezprozvannaya S, Raguimova ON, Cho EE, Vidal AH, Robia SL, Bassel-Duby R, Olson EN
eLife 2018 Oct 9;7
eLife 2018 Oct 9;7
Facilitation of ischaemia-induced ventricular fibrillation by catecholamines is mediated by β(1) and β(2) agonism in the rat heart in vitro.
Wilder CDE, Pavlaki N, Dursun T, Gyimah P, Caldwell-Dunn E, Ranieri A, Lewis HR, Curtis MJ
British journal of pharmacology 2018 May;175(10):1669-1690
British journal of pharmacology 2018 May;175(10):1669-1690
Conditional ablation and conditional rescue models for Casq2 elucidate the role of development and of cell-type specific expression of Casq2 in the CPVT2 phenotype.
Flores DJ, Duong T, Brandenberger LO, Mitra A, Shirali A, Johnson JC, Springer D, Noguchi A, Yu ZX, Ebert SN, Ludwig A, Knollmann BC, Levin MD, Pfeifer K
Human molecular genetics 2018 May 1;27(9):1533-1544
Human molecular genetics 2018 May 1;27(9):1533-1544
Inflammation leads through PGE/EP(3) signaling to HDAC5/MEF2-dependent transcription in cardiac myocytes.
Tóth AD, Schell R, Lévay M, Vettel C, Theis P, Haslinger C, Alban F, Werhahn S, Frischbier L, Krebs-Haupenthal J, Thomas D, Gröne HJ, Avkiran M, Katus HA, Wieland T, Backs J
EMBO molecular medicine 2018 Jul;10(7)
EMBO molecular medicine 2018 Jul;10(7)
A secretory pathway kinase regulates sarcoplasmic reticulum Ca(2+) homeostasis and protects against heart failure.
Pollak AJ, Liu C, Gudlur A, Mayfield JE, Dalton ND, Gu Y, Chen J, Heller Brown J, Hogan PG, Wiley SE, Peterson KL, Dixon JE
eLife 2018 Dec 6;7
eLife 2018 Dec 6;7
A chemical chaperone improves muscle function in mice with a RyR1 mutation.
Lee CS, Hanna AD, Wang H, Dagnino-Acosta A, Joshi AD, Knoblauch M, Xia Y, Georgiou DK, Xu J, Long C, Amano H, Reynolds C, Dong K, Martin JC, Lagor WR, Rodney GG, Sahin E, Sewry C, Hamilton SL
Nature communications 2017 Mar 24;8:14659
Nature communications 2017 Mar 24;8:14659
Calcium dysregulation, functional calpainopathy, and endoplasmic reticulum stress in sporadic inclusion body myositis.
Amici DR, Pinal-Fernandez I, Mázala DA, Lloyd TE, Corse AM, Christopher-Stine L, Mammen AL, Chin ER
Acta neuropathologica communications 2017 Mar 22;5(1):24
Acta neuropathologica communications 2017 Mar 22;5(1):24
T-tubule remodelling disturbs localized β2-adrenergic signalling in rat ventricular myocytes during the progression of heart failure.
Schobesberger S, Wright P, Tokar S, Bhargava A, Mansfield C, Glukhov AV, Poulet C, Buzuk A, Monszpart A, Sikkel M, Harding SE, Nikolaev VO, Lyon AR, Gorelik J
Cardiovascular research 2017 Jun 1;113(7):770-782
Cardiovascular research 2017 Jun 1;113(7):770-782
The TRPM4 channel is functionally important for the beneficial cardiac remodeling induced by endurance training.
Gueffier M, Zintz J, Lambert K, Finan A, Aimond F, Chakouri N, Hédon C, Granier M, Launay P, Thireau J, Richard S, Demion M
Journal of muscle research and cell motility 2017 Feb;38(1):3-16
Journal of muscle research and cell motility 2017 Feb;38(1):3-16
Gene-Targeted Mice with the Human Troponin T R141W Mutation Develop Dilated Cardiomyopathy with Calcium Desensitization.
Ramratnam M, Salama G, Sharma RK, Wang DW, Smith SH, Banerjee SK, Huang XN, Gifford LM, Pruce ML, Gabris BE, Saba S, Shroff SG, Ahmad F
PloS one 2016;11(12):e0167681
PloS one 2016;11(12):e0167681
Genotype-Dependent and -Independent Calcium Signaling Dysregulation in Human Hypertrophic Cardiomyopathy.
Helms AS, Alvarado FJ, Yob J, Tang VT, Pagani F, Russell MW, Valdivia HH, Day SM
Circulation 2016 Nov 29;134(22):1738-1748
Circulation 2016 Nov 29;134(22):1738-1748
Acyl CoA synthetase-1 links facilitated long chain fatty acid uptake to intracellular metabolic trafficking differently in hearts of male versus female mice.
Goldenberg JR, Wang X, Lewandowski ED
Journal of molecular and cellular cardiology 2016 May;94:1-9
Journal of molecular and cellular cardiology 2016 May;94:1-9
The angiotensin receptor-associated protein Atrap is a stimulator of the cardiac Ca2+-ATPase SERCA2a.
Mederle K, Gess B, Pluteanu F, Plackic J, Tiefenbach KJ, Grill A, Kockskämper J, Castrop H
Cardiovascular research 2016 Jun 1;110(3):359-70
Cardiovascular research 2016 Jun 1;110(3):359-70
Heterozygous deletion of sarcolipin maintains normal cardiac function.
Shimura D, Kusakari Y, Sasano T, Nakashima Y, Nakai G, Jiao Q, Jin M, Yokota T, Ishikawa Y, Nakano A, Goda N, Minamisawa S
American journal of physiology. Heart and circulatory physiology 2016 Jan 1;310(1):H92-103
American journal of physiology. Heart and circulatory physiology 2016 Jan 1;310(1):H92-103
Protein Phosphatase 1c Associated with the Cardiac Sodium Calcium Exchanger 1 Regulates Its Activity by Dephosphorylating Serine 68-phosphorylated Phospholemman.
Hafver TL, Hodne K, Wanichawan P, Aronsen JM, Dalhus B, Lunde PK, Lunde M, Martinsen M, Enger UH, Fuller W, Sjaastad I, Louch WE, Sejersted OM, Carlson CR
The Journal of biological chemistry 2016 Feb 26;291(9):4561-79
The Journal of biological chemistry 2016 Feb 26;291(9):4561-79
The maintenance ability and Ca(2+) availability of skeletal muscle are enhanced by sildenafil.
Huang M, Lee KJ, Kim KJ, Ahn MK, Cho CH, Kim DH, Lee EH
Experimental & molecular medicine 2016 Dec 9;48(12):e278
Experimental & molecular medicine 2016 Dec 9;48(12):e278
Cannabinoid signalling inhibits sarcoplasmic Ca(2+) release and regulates excitation-contraction coupling in mammalian skeletal muscle.
Oláh T, Bodnár D, Tóth A, Vincze J, Fodor J, Reischl B, Kovács A, Ruzsnavszky O, Dienes B, Szentesi P, Friedrich O, Csernoch L
The Journal of physiology 2016 Dec 15;594(24):7381-7398
The Journal of physiology 2016 Dec 15;594(24):7381-7398
Development of a high-affinity peptide that prevents phospholemman (PLM) inhibition of the sodium/calcium exchanger 1 (NCX1).
Wanichawan P, Hodne K, Hafver TL, Lunde M, Martinsen M, Louch WE, Sejersted OM, Carlson CR
The Biochemical journal 2016 Aug 1;473(15):2413-23
The Biochemical journal 2016 Aug 1;473(15):2413-23
Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding.
De Andrade PB, Neff LA, Strosova MK, Arsenijevic D, Patthey-Vuadens O, Scapozza L, Montani JP, Ruegg UT, Dulloo AG, Dorchies OM
Frontiers in physiology 2015;6:254
Frontiers in physiology 2015;6:254
Ca(2+) permeation and/or binding to CaV1.1 fine-tunes skeletal muscle Ca(2+) signaling to sustain muscle function.
Lee CS, Dagnino-Acosta A, Yarotskyy V, Hanna A, Lyfenko A, Knoblauch M, Georgiou DK, Poché RA, Swank MW, Long C, Ismailov II, Lanner J, Tran T, Dong K, Rodney GG, Dickinson ME, Beeton C, Zhang P, Dirksen RT, Hamilton SL
Skeletal muscle 2015;5:4
Skeletal muscle 2015;5:4
Effectiveness of gene delivery systems for pluripotent and differentiated cells.
Rapti K, Stillitano F, Karakikes I, Nonnenmacher M, Weber T, Hulot JS, Hajjar RJ
Molecular therapy. Methods & clinical development 2015;2:14067
Molecular therapy. Methods & clinical development 2015;2:14067
The Effect of SERCA1b Silencing on the Differentiation and Calcium Homeostasis of C2C12 Skeletal Muscle Cells.
Tóth A, Fodor J, Vincze J, Oláh T, Juhász T, Zákány R, Csernoch L, Zádor E
PloS one 2015;10(4):e0123583
PloS one 2015;10(4):e0123583
Suppression of Early and Late Afterdepolarizations by Heterozygous Knockout of the Na+/Ca2+ Exchanger in a Murine Model.
Bögeholz N, Pauls P, Bauer BK, Schulte JS, Dechering DG, Frommeyer G, Kirchhefer U, Goldhaber JI, Müller FU, Eckardt L, Pott C
Circulation. Arrhythmia and electrophysiology 2015 Oct;8(5):1210-8
Circulation. Arrhythmia and electrophysiology 2015 Oct;8(5):1210-8
Expression of calcium-buffering proteins in rat intrinsic laryngeal muscles.
Ferretti R, Marques MJ, Khurana TS, Santo Neto H
Physiological reports 2015 Jun;3(6)
Physiological reports 2015 Jun;3(6)
Sox9 expression in canine epithelial skin tumors.
Fantinato E, Milani L, Sironi G
European journal of histochemistry : EJH 2015 Jul 9;59(3):2514
European journal of histochemistry : EJH 2015 Jul 9;59(3):2514
Metabolic efficiency promotes protection from pressure overload in hearts expressing slow skeletal troponin I.
Carley AN, Taglieri DM, Bi J, Solaro RJ, Lewandowski ED
Circulation. Heart failure 2015 Jan;8(1):119-27
Circulation. Heart failure 2015 Jan;8(1):119-27
Changes in the cardiac metabolome caused by perhexiline treatment in a mouse model of hypertrophic cardiomyopathy.
Gehmlich K, Dodd MS, Allwood JW, Kelly M, Bellahcene M, Lad HV, Stockenhuber A, Hooper C, Ashrafian H, Redwood CS, Carrier L, Dunn WB
Molecular bioSystems 2015 Feb;11(2):564-73
Molecular bioSystems 2015 Feb;11(2):564-73
p11 modulates calcium handling through 5-HT₄R pathway in rat ventricular cardiomyocytes.
Meschin P, Demion M, Cazorla O, Finan A, Thireau J, Richard S, Lacampagne A
Cell calcium 2015 Dec;58(6):549-57
Cell calcium 2015 Dec;58(6):549-57
Mitsugumin 56 (hedgehog acyltransferase-like) is a sarcoplasmic reticulum-resident protein essential for postnatal muscle maturation.
Van B, Nishi M, Komazaki S, Ichimura A, Kakizawa S, Nakanaga K, Aoki J, Park KH, Ma J, Ueyama T, Ogata T, Maruyama N, Takeshima H
FEBS letters 2015 Apr 28;589(10):1095-104
FEBS letters 2015 Apr 28;589(10):1095-104
Regional ion channel gene expression heterogeneity and ventricular fibrillation dynamics in human hearts.
Sivagangabalan G, Nazzari H, Bignolais O, Maguy A, Naud P, Farid T, Massé S, Gaborit N, Varro A, Nair K, Backx P, Vigmond E, Nattel S, Demolombe S, Nanthakumar K
PloS one 2014;9(1):e82179
PloS one 2014;9(1):e82179
Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age.
Denegri M, Bongianino R, Lodola F, Boncompagni S, De Giusti VC, Avelino-Cruz JE, Liu N, Persampieri S, Curcio A, Esposito F, Pietrangelo L, Marty I, Villani L, Moyaho A, Baiardi P, Auricchio A, Protasi F, Napolitano C, Priori SG
Circulation 2014 Jun 24;129(25):2673-81
Circulation 2014 Jun 24;129(25):2673-81
Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation.
Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D
Circulation 2014 Jan 14;129(2):145-156
Circulation 2014 Jan 14;129(2):145-156
The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias.
Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O'Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song LS, Chen SR
Nature medicine 2014 Feb;20(2):184-92
Nature medicine 2014 Feb;20(2):184-92
Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release.
Chiang DY, Kongchan N, Beavers DL, Alsina KM, Voigt N, Neilson JR, Jakob H, Martin JF, Dobrev D, Wehrens XH, Li N
Circulation. Arrhythmia and electrophysiology 2014 Dec;7(6):1214-22
Circulation. Arrhythmia and electrophysiology 2014 Dec;7(6):1214-22
Molecular basis of calpain cleavage and inactivation of the sodium-calcium exchanger 1 in heart failure.
Wanichawan P, Hafver TL, Hodne K, Aronsen JM, Lunde IG, Dalhus B, Lunde M, Kvaløy H, Louch WE, Tønnessen T, Sjaastad I, Sejersted OM, Carlson CR
The Journal of biological chemistry 2014 Dec 5;289(49):33984-98
The Journal of biological chemistry 2014 Dec 5;289(49):33984-98
The PEG-switch assay: a fast semi-quantitative method to determine protein reversible cysteine oxidation.
Burgoyne JR, Oviosu O, Eaton P
Journal of pharmacological and toxicological methods 2013 Nov-Dec;68(3):297-301
Journal of pharmacological and toxicological methods 2013 Nov-Dec;68(3):297-301
Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart.
Strand ME, Herum KM, Rana ZA, Skrbic B, Askevold ET, Dahl CP, Vistnes M, Hasic A, Kvaløy H, Sjaastad I, Carlson CR, Tønnessen T, Gullestad L, Christensen G, Lunde IG
The FEBS journal 2013 May;280(10):2228-47
The FEBS journal 2013 May;280(10):2228-47
Noncanonical EF-hand motif strategically delays Ca2+ buffering to enhance cardiac performance.
Wang W, Barnabei MS, Asp ML, Heinis FI, Arden E, Davis J, Braunlin E, Li Q, Davis JP, Potter JD, Metzger JM
Nature medicine 2013 Mar;19(3):305-12
Nature medicine 2013 Mar;19(3):305-12
The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy.
Dorchies OM, Reutenauer-Patte J, Dahmane E, Ismail HM, Petermann O, Patthey- Vuadens O, Comyn SA, Gayi E, Piacenza T, Handa RJ, Décosterd LA, Ruegg UT
The American journal of pathology 2013 Feb;182(2):485-504
The American journal of pathology 2013 Feb;182(2):485-504
Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy.
Schneider JS, Shanmugam M, Gonzalez JP, Lopez H, Gordan R, Fraidenraich D, Babu GJ
Journal of muscle research and cell motility 2013 Dec;34(5-6):349-56
Journal of muscle research and cell motility 2013 Dec;34(5-6):349-56
Comparative differential proteomic profiles of nonfailing and failing hearts after in vivo thoracic aortic constriction in mice overexpressing FKBP12.6.
Prévilon M, Le Gall M, Chafey P, Federeci C, Pezet M, Clary G, Broussard C, François G, Mercadier JJ, Rouet-Benzineb P
Physiological reports 2013 Aug;1(3):e00039
Physiological reports 2013 Aug;1(3):e00039
Knockout of the Na,K-ATPase α2-isoform in cardiac myocytes delays pressure overload-induced cardiac dysfunction.
Rindler TN, Lasko VM, Nieman ML, Okada M, Lorenz JN, Lingrel JB
American journal of physiology. Heart and circulatory physiology 2013 Apr 15;304(8):H1147-58
American journal of physiology. Heart and circulatory physiology 2013 Apr 15;304(8):H1147-58
Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy.
Drawnel FM, Wachten D, Molkentin JD, Maillet M, Aronsen JM, Swift F, Sjaastad I, Liu N, Catalucci D, Mikoshiba K, Hisatsune C, Okkenhaug H, Andrews SR, Bootman MD, Roderick HL
The Journal of cell biology 2012 Nov 26;199(5):783-98
The Journal of cell biology 2012 Nov 26;199(5):783-98
Functional scaffold-free 3-D cardiac microtissues: a novel model for the investigation of heart cells.
Desroches BR, Zhang P, Choi BR, King ME, Maldonado AE, Li W, Rago A, Liu G, Nath N, Hartmann KM, Yang B, Koren G, Morgan JR, Mende U
American journal of physiology. Heart and circulatory physiology 2012 May 15;302(10):H2031-42
American journal of physiology. Heart and circulatory physiology 2012 May 15;302(10):H2031-42
CREB critically regulates action potential shape and duration in the adult mouse ventricle.
Schulte JS, Seidl MD, Nunes F, Freese C, Schneider M, Schmitz W, Müller FU
American journal of physiology. Heart and circulatory physiology 2012 May 15;302(10):H1998-2007
American journal of physiology. Heart and circulatory physiology 2012 May 15;302(10):H1998-2007
Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes.
Sassi Y, Abi-Gerges A, Fauconnier J, Mougenot N, Reiken S, Haghighi K, Kranias EG, Marks AR, Lacampagne A, Engelhardt S, Hatem SN, Lompre AM, Hulot JS
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2012 Mar;26(3):1009-17
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2012 Mar;26(3):1009-17
Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias.
Denegri M, Avelino-Cruz JE, Boncompagni S, De Simone SA, Auricchio A, Villani L, Volpe P, Protasi F, Napolitano C, Priori SG
Circulation research 2012 Mar 2;110(5):663-8
Circulation research 2012 Mar 2;110(5):663-8
Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction.
Gurha P, Abreu-Goodger C, Wang T, Ramirez MO, Drumond AL, van Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, Kelm RJ Jr, Reddy AK, Taffet GE, Bradley A, Wehrens XH, Entman ML, Rodriguez A
Circulation 2012 Jun 5;125(22):2751-61
Circulation 2012 Jun 5;125(22):2751-61
Functional evidence for an active role of B-type natriuretic peptide in cardiac remodelling and pro-arrhythmogenicity.
Thireau J, Karam S, Fauconnier J, Roberge S, Cassan C, Cazorla O, Aimond F, Lacampagne A, Babuty D, Richard S
Cardiovascular research 2012 Jul 1;95(1):59-68
Cardiovascular research 2012 Jul 1;95(1):59-68
Role of Junctin protein interactions in cellular dynamics of calsequestrin polymer upon calcium perturbation.
Lee KW, Maeng JS, Choi JY, Lee YR, Hwang CY, Park SS, Park HK, Chung BH, Lee SG, Kim YS, Jeon H, Eom SH, Kang C, Kim DH, Kwon KS
The Journal of biological chemistry 2012 Jan 13;287(3):1679-87
The Journal of biological chemistry 2012 Jan 13;287(3):1679-87
Immortalization of bone marrow-derived porcine mesenchymal stem cells and their differentiation into cells expressing cardiac phenotypic markers.
Moscoso I, Rodriguez-Barbosa JI, Barallobre-Barreiro J, Anon P, Domenech N
Journal of tissue engineering and regenerative medicine 2012 Aug;6(8):655-65
Journal of tissue engineering and regenerative medicine 2012 Aug;6(8):655-65
4D super-resolution microscopy with conventional fluorophores and single wavelength excitation in optically thick cells and tissues.
Baddeley D, Crossman D, Rossberger S, Cheyne JE, Montgomery JM, Jayasinghe ID, Cremer C, Cannell MB, Soeller C
PloS one 2011;6(5):e20645
PloS one 2011;6(5):e20645
Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway.
Finsen AV, Lunde IG, Sjaastad I, Østli EK, Lyngra M, Jarstadmarken HO, Hasic A, Nygård S, Wilcox-Adelman SA, Goetinck PF, Lyberg T, Skrbic B, Florholmen G, Tønnessen T, Louch WE, Djurovic S, Carlson CR, Christensen G
PloS one 2011;6(12):e28302
PloS one 2011;6(12):e28302
Differential effect of calsequestrin ablation on structure and function of fast and slow skeletal muscle fibers.
Paolini C, Quarta M, D'Onofrio L, Reggiani C, Protasi F
Journal of biomedicine & biotechnology 2011;2011:634075
Journal of biomedicine & biotechnology 2011;2011:634075
Organic cation transporter 3: expression in failing and nonfailing human heart and functional characterization.
Solbach TF, Grube M, Fromm MF, Zolk O
Journal of cardiovascular pharmacology 2011 Oct;58(4):409-17
Journal of cardiovascular pharmacology 2011 Oct;58(4):409-17
Catecholaminergic-induced arrhythmias in failing cardiomyocytes associated with human HRCS96A variant overexpression.
Han P, Cai W, Wang Y, Lam CK, Arvanitis DA, Singh VP, Chen S, Zhang H, Zhang R, Cheng H, Kranias EG
American journal of physiology. Heart and circulatory physiology 2011 Oct;301(4):H1588-95
American journal of physiology. Heart and circulatory physiology 2011 Oct;301(4):H1588-95
Knockout of the Na,K-ATPase α₂-isoform in the cardiovascular system does not alter basal blood pressure but prevents ACTH-induced hypertension.
Rindler TN, Dostanic I, Lasko VM, Nieman ML, Neumann JC, Lorenz JN, Lingrel JB
American journal of physiology. Heart and circulatory physiology 2011 Oct;301(4):H1396-404
American journal of physiology. Heart and circulatory physiology 2011 Oct;301(4):H1396-404
FKBP12.6 mice display temporal gender differences in cardiac Ca(2+)-signalling phenotype upon chronic pressure overload.
Prévilon M, Pezet M, Semprez F, Mercadier JJ, Rouet-Benzineb P
Canadian journal of physiology and pharmacology 2011 Nov;89(11):769-82
Canadian journal of physiology and pharmacology 2011 Nov;89(11):769-82
Antioxidant network expression abrogates oxidative posttranslational modifications in mice.
Mital R, Zhang W, Cai M, Huttinger ZM, Goodman LA, Wheeler DG, Ziolo MT, Dwyer KM, d'Apice AJ, Zweier JL, He G, Cowan PJ, Gumina RJ
American journal of physiology. Heart and circulatory physiology 2011 May;300(5):H1960-70
American journal of physiology. Heart and circulatory physiology 2011 May;300(5):H1960-70
Prevention of ventricular arrhythmia and calcium dysregulation in a catecholaminergic polymorphic ventricular tachycardia mouse model carrying calsequestrin-2 mutation.
Alcalai R, Wakimoto H, Arad M, Planer D, Konno T, Wang L, Seidman JG, Seidman CE, Berul CI
Journal of cardiovascular electrophysiology 2011 Mar;22(3):316-24
Journal of cardiovascular electrophysiology 2011 Mar;22(3):316-24
Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function.
Russ DW, Grandy JS, Toma K, Ward CW
Acta physiologica (Oxford, England) 2011 Mar;201(3):391-403
Acta physiologica (Oxford, England) 2011 Mar;201(3):391-403
Conserved expression and functions of PDE4 in rodent and human heart.
Richter W, Xie M, Scheitrum C, Krall J, Movsesian MA, Conti M
Basic research in cardiology 2011 Mar;106(2):249-62
Basic research in cardiology 2011 Mar;106(2):249-62
Impaired β-adrenergic responsiveness accentuates dysfunctional excitation-contraction coupling in an ovine model of tachypacing-induced heart failure.
Briston SJ, Caldwell JL, Horn MA, Clarke JD, Richards MA, Greensmith DJ, Graham HK, Hall MC, Eisner DA, Dibb KM, Trafford AW
The Journal of physiology 2011 Mar 15;589(Pt 6):1367-82
The Journal of physiology 2011 Mar 15;589(Pt 6):1367-82
SR-targeted CaMKII inhibition improves SR Ca²+ handling, but accelerates cardiac remodeling in mice overexpressing CaMKIIδC.
Huke S, Desantiago J, Kaetzel MA, Mishra S, Brown JH, Dedman JR, Bers DM
Journal of molecular and cellular cardiology 2011 Jan;50(1):230-8
Journal of molecular and cellular cardiology 2011 Jan;50(1):230-8
STAT subtype specificity and ischemic preconditioning in mice: is STAT-3 enough?
Goodman MD, Koch SE, Afzal MR, Butler KL
American journal of physiology. Heart and circulatory physiology 2011 Feb;300(2):H522-6
American journal of physiology. Heart and circulatory physiology 2011 Feb;300(2):H522-6
Couplons in rat atria form distinct subgroups defined by their molecular partners.
Schulson MN, Scriven DR, Fletcher P, Moore ED
Journal of cell science 2011 Apr 1;124(Pt 7):1167-74
Journal of cell science 2011 Apr 1;124(Pt 7):1167-74
Caspase-dependent protein phosphatase 2A activation contributes to endotoxin-induced cardiomyocyte contractile dysfunction.
Neviere R, Hassoun SM, Decoster B, Bouazza Y, Montaigne D, Maréchal X, Marciniak C, Marchetti P, Lancel S
Critical care medicine 2010 Oct;38(10):2031-6
Critical care medicine 2010 Oct;38(10):2031-6
Mitochondrial uncoupling downregulates calsequestrin expression and reduces SR Ca2+ stores in cardiomyocytes.
Hänninen SL, Ronkainen JJ, Leskinen H, Tavi P
Cardiovascular research 2010 Oct 1;88(1):75-82
Cardiovascular research 2010 Oct 1;88(1):75-82
Superior calcium homeostasis of extraocular muscles.
Zeiger U, Mitchell CH, Khurana TS
Experimental eye research 2010 Nov;91(5):613-22
Experimental eye research 2010 Nov;91(5):613-22
Sequential alterations in Akt, GSK3β, and calcineurin signalling in the mouse left ventricle after thoracic aortic constriction.
Prévilon M, Pezet M, Dachez C, Mercadier JJ, Rouet-Benzineb P
Canadian journal of physiology and pharmacology 2010 Nov;88(11):1093-101
Canadian journal of physiology and pharmacology 2010 Nov;88(11):1093-101
S165F mutation of junctophilin 2 affects Ca2+ signalling in skeletal muscle.
Woo JS, Hwang JH, Ko JK, Weisleder N, Kim DH, Ma J, Lee EH
The Biochemical journal 2010 Mar 15;427(1):125-34
The Biochemical journal 2010 Mar 15;427(1):125-34
Carbon monoxide pollution promotes cardiac remodeling and ventricular arrhythmia in healthy rats.
Andre L, Boissière J, Reboul C, Perrier R, Zalvidea S, Meyer G, Thireau J, Tanguy S, Bideaux P, Hayot M, Boucher F, Obert P, Cazorla O, Richard S
American journal of respiratory and critical care medicine 2010 Mar 15;181(6):587-95
American journal of respiratory and critical care medicine 2010 Mar 15;181(6):587-95
Chronic heart rate reduction with ivabradine improves systolic function of the reperfused heart through a dual mechanism involving a direct mechanical effect and a long-term increase in FKBP12/12.6 expression.
Couvreur N, Tissier R, Pons S, Chetboul V, Gouni V, Bruneval P, Mandet C, Pouchelon JL, Berdeaux A, Ghaleh B
European heart journal 2010 Jun;31(12):1529-37
European heart journal 2010 Jun;31(12):1529-37
A new regulation of IL-6 production in adult cardiomyocytes by beta-adrenergic and IL-1 beta receptors and induction of cellular hypertrophy by IL-6 trans-signalling.
Szabo-Fresnais N, Lefebvre F, Germain A, Fischmeister R, Pomérance M
Cellular signalling 2010 Jul;22(7):1143-52
Cellular signalling 2010 Jul;22(7):1143-52
Postnatal development of mouse heart: formation of energetic microdomains.
Piquereau J, Novotova M, Fortin D, Garnier A, Ventura-Clapier R, Veksler V, Joubert F
The Journal of physiology 2010 Jul 1;588(Pt 13):2443-54
The Journal of physiology 2010 Jul 1;588(Pt 13):2443-54
The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering.
Kalyanasundaram A, Bal NC, Franzini-Armstrong C, Knollmann BC, Periasamy M
The Journal of biological chemistry 2010 Jan 29;285(5):3076-83
The Journal of biological chemistry 2010 Jan 29;285(5):3076-83
Calcium-binding proteins in skeletal muscles of the mdx mice: potential role in the pathogenesis of Duchenne muscular dystrophy.
Pertille A, de Carvalho CL, Matsumura CY, Neto HS, Marques MJ
International journal of experimental pathology 2010 Feb;91(1):63-71
International journal of experimental pathology 2010 Feb;91(1):63-71
Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice.
Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH
Circulation research 2010 Feb 5;106(2):354-62
Circulation research 2010 Feb 5;106(2):354-62
Diabetes-related defects in sarcoplasmic Ca2+ release are prevented by inactivation of G(alpha)11 and G(alpha)q in murine cardiomyocytes.
Hoyer DP, Grönke S, Frank KF, Addicks K, Wettschureck N, Offermanns S, Erdmann E, Reuter H
Molecular and cellular biochemistry 2010 Aug;341(1-2):235-44
Molecular and cellular biochemistry 2010 Aug;341(1-2):235-44
Effects of AT1- and beta-adrenergic receptor antagonists on TGF-beta1-induced fibrosis in transgenic mice.
Seeland U, Schäffer A, Selejan S, Hohl M, Reil JC, Müller P, Rosenkranz S, Böhm M
European journal of clinical investigation 2009 Oct;39(10):851-9
European journal of clinical investigation 2009 Oct;39(10):851-9
Altered contractility of skeletal muscle in mice deficient in titin's M-band region.
Ottenheijm CA, Hidalgo C, Rost K, Gotthardt M, Granzier H
Journal of molecular biology 2009 Oct 16;393(1):10-26
Journal of molecular biology 2009 Oct 16;393(1):10-26
Diltiazem and verapamil protect dystrophin-deficient muscle fibers of MDX mice from degeneration: a potential role in calcium buffering and sarcolemmal stability.
Matsumura CY, Pertille A, Albuquerque TC, Santo Neto H, Marques MJ
Muscle & nerve 2009 Feb;39(2):167-76
Muscle & nerve 2009 Feb;39(2):167-76
Reduced expression of sarcalumenin and related Ca2+ -regulatory proteins in aged rat skeletal muscle.
O'Connell K, Gannon J, Doran P, Ohlendieck K
Experimental gerontology 2008 Oct;43(10):958-61
Experimental gerontology 2008 Oct;43(10):958-61
Single histidine-substituted cardiac troponin I confers protection from age-related systolic and diastolic dysfunction.
Palpant NJ, Day SM, Herron TJ, Converso KL, Metzger JM
Cardiovascular research 2008 Nov 1;80(2):209-18
Cardiovascular research 2008 Nov 1;80(2):209-18
Cardiac-directed parvalbumin transgene expression in mice shows marked heart rate dependence of delayed Ca2+ buffering action.
Day SM, Coutu P, Wang W, Herron T, Turner I, Shillingford M, Lacross NC, Converso KL, Piao L, Li J, Lopatin AN, Metzger JM
Physiological genomics 2008 May 13;33(3):312-22
Physiological genomics 2008 May 13;33(3):312-22
Enhanced calcium cycling and contractile function in transgenic hearts expressing constitutively active G alpha o* protein.
Zhu M, Gach AA, Liu G, Xu X, Lim CC, Zhang JX, Mao L, Chuprun K, Koch WJ, Liao R, Koren G, Blaxall BC, Mende U
American journal of physiology. Heart and circulatory physiology 2008 Mar;294(3):H1335-47
American journal of physiology. Heart and circulatory physiology 2008 Mar;294(3):H1335-47
S100A1 deficiency results in prolonged ventricular repolarization in response to sympathetic activation.
Ackermann GE, Domenighetti AA, Deten A, Bonath I, Marenholz I, Pedrazzini T, Erne P, Heizmann CW
General physiology and biophysics 2008 Jun;27(2):127-42
General physiology and biophysics 2008 Jun;27(2):127-42
Limited functional and metabolic improvements in hypertrophic and healthy rat heart overexpressing the skeletal muscle isoform of SERCA1 by adenoviral gene transfer in vivo.
O'Donnell JM, Fields A, Xu X, Chowdhury SA, Geenen DL, Bi J
American journal of physiology. Heart and circulatory physiology 2008 Dec;295(6):H2483-94
American journal of physiology. Heart and circulatory physiology 2008 Dec;295(6):H2483-94
Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model.
Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori SG
Circulation research 2008 Aug 1;103(3):298-306
Circulation research 2008 Aug 1;103(3):298-306
Interstitial remodeling in beta1-adrenergic receptor transgenic mice.
Seeland U, Selejan S, Engelhardt S, Müller P, Lohse MJ, Böhm M
Basic research in cardiology 2007 Mar;102(2):183-93
Basic research in cardiology 2007 Mar;102(2):183-93
Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes.
Fu M, Wu M, Wang JF, Qiao YJ, Wang Z
Biochemical and biophysical research communications 2007 Mar 23;354(4):929-36
Biochemical and biophysical research communications 2007 Mar 23;354(4):929-36
Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia.
di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG
Circulation 2006 Sep 5;114(10):1012-9
Circulation 2006 Sep 5;114(10):1012-9
High intracellular Na+ preserves myocardial function at low heart rates in isolated myocardium from failing hearts.
Schillinger W, Teucher N, Christians C, Kohlhaas M, Sossalla S, Van Nguyen P, Schmidt AG, Schunck O, Nebendahl K, Maier LS, Zeitz O, Hasenfuss G
European journal of heart failure 2006 Nov;8(7):673-80
European journal of heart failure 2006 Nov;8(7):673-80
Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death.
Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S
Circulation research 2006 May 12;98(9):1151-8
Circulation research 2006 May 12;98(9):1151-8
LAMP-2 deficient mice show depressed cardiac contractile function without significant changes in calcium handling.
Stypmann J, Janssen PM, Prestle J, Engelen MA, Kögler H, Lüllmann-Rauch R, Eckardt L, von Figura K, Landgrebe J, Mleczko A, Saftig P
Basic research in cardiology 2006 Jul;101(4):281-91
Basic research in cardiology 2006 Jul;101(4):281-91
Association of RhoGDIalpha with Rac1 GTPase mediates free radical production during myocardial hypertrophy.
Custodis F, Eberl M, Kilter H, Böhm M, Laufs U
Cardiovascular research 2006 Jul 15;71(2):342-51
Cardiovascular research 2006 Jul 15;71(2):342-51
Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction.
Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O
Stem cells (Dayton, Ohio) 2006 Feb;24(2):236-45
Stem cells (Dayton, Ohio) 2006 Feb;24(2):236-45
Increased susceptibility to isoproterenol-induced cardiac hypertrophy and impaired weight gain in mice lacking the histidine-rich calcium-binding protein.
Jaehnig EJ, Heidt AB, Greene SB, Cornelissen I, Black BL
Molecular and cellular biology 2006 Dec;26(24):9315-26
Molecular and cellular biology 2006 Dec;26(24):9315-26
Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure.
Rothermel BA, Berenji K, Tannous P, Kutschke W, Dey A, Nolan B, Yoo KD, Demetroulis E, Gimbel M, Cabuay B, Karimi M, Hill JA
Physiological genomics 2005 Sep 21;23(1):18-27
Physiological genomics 2005 Sep 21;23(1):18-27
Myotonic dystrophy protein kinase phosphorylates phospholamban and regulates calcium uptake in cardiomyocyte sarcoplasmic reticulum.
Kaliman P, Catalucci D, Lam JT, Kondo R, Gutiérrez JC, Reddy S, Palacín M, Zorzano A, Chien KR, Ruiz-Lozano P
The Journal of biological chemistry 2005 Mar 4;280(9):8016-21
The Journal of biological chemistry 2005 Mar 4;280(9):8016-21
Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies.
Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacène E, Fromes Y, Toussaint M, Mura AM, Keller DI, Amthor H, Isnard R, Malissen M, Schwartz K, Bonne G
Human molecular genetics 2005 Jan 1;14(1):155-69
Human molecular genetics 2005 Jan 1;14(1):155-69
Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes.
Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Williams SC, Gyorke S
Circulation research 2005 Apr 1;96(6):651-8
Circulation research 2005 Apr 1;96(6):651-8
Phosphorylation-status of phospholamban and calsequestrin modifies their affinity towards commonly used antibodies.
Huke S, Periasamy M
Journal of molecular and cellular cardiology 2004 Sep;37(3):795-9
Journal of molecular and cellular cardiology 2004 Sep;37(3):795-9
Regulation of dihydropyridine receptor gene expression in mouse skeletal muscles by stretch and disuse.
Radzyukevich TL, Heiny JA
American journal of physiology. Cell physiology 2004 Nov;287(5):C1445-52
American journal of physiology. Cell physiology 2004 Nov;287(5):C1445-52
The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm.
Radzyukevich TL, Moseley AE, Shelly DA, Redden GA, Behbehani MM, Lingrel JB, Paul RJ, Heiny JA
American journal of physiology. Cell physiology 2004 Nov;287(5):C1300-10
American journal of physiology. Cell physiology 2004 Nov;287(5):C1300-10
Human homozygous R403W mutant cardiac myosin presents disproportionate enhancement of mechanical and enzymatic properties.
Keller DI, Coirault C, Rau T, Cheav T, Weyand M, Amann K, Lecarpentier Y, Richard P, Eschenhagen T, Carrier L
Journal of molecular and cellular cardiology 2004 Mar;36(3):355-62
Journal of molecular and cellular cardiology 2004 Mar;36(3):355-62
Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity.
Schultz Jel J, Glascock BJ, Witt SA, Nieman ML, Nattamai KJ, Liu LH, Lorenz JN, Shull GE, Kimball TR, Periasamy M
American journal of physiology. Heart and circulatory physiology 2004 Mar;286(3):H1146-53
American journal of physiology. Heart and circulatory physiology 2004 Mar;286(3):H1146-53
Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin.
Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Györke S
Circulation research 2004 Mar 5;94(4):471-7
Circulation research 2004 Mar 5;94(4):471-7
Phospholamban gene ablation improves calcium transients but not cardiac function in a heart failure model.
Janczewski AM, Zahid M, Lemster BH, Frye CS, Gibson G, Higuchi Y, Kranias EG, Feldman AM, McTiernan CF
Cardiovascular research 2004 Jun 1;62(3):468-80
Cardiovascular research 2004 Jun 1;62(3):468-80
Effects of chronic endothelin-1 stimulation on cardiac myocyte contractile function.
Zolk O, Münzel F, Eschenhagen T
American journal of physiology. Heart and circulatory physiology 2004 Apr;286(4):H1248-57
American journal of physiology. Heart and circulatory physiology 2004 Apr;286(4):H1248-57
Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia.
Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S
Proceedings of the National Academy of Sciences of the United States of America 2003 Sep 30;100(20):11759-64
Proceedings of the National Academy of Sciences of the United States of America 2003 Sep 30;100(20):11759-64
Na(+)-Ca(2+) exchanger overexpression predisposes to reactive oxygen species-induced injury.
Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kögler H
Cardiovascular research 2003 Nov 1;60(2):404-12
Cardiovascular research 2003 Nov 1;60(2):404-12
Na(+)-Ca(2+) exchanger overexpression predisposes to reactive oxygen species-induced injury.
Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kögler H
Cardiovascular research 2003 Nov 1;60(2):404-12
Cardiovascular research 2003 Nov 1;60(2):404-12
Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17.
Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR
The Journal of biological chemistry 2003 Jul 4;278(27):25063-71
The Journal of biological chemistry 2003 Jul 4;278(27):25063-71
Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol.
Chen J, Petranka J, Yamamura K, London RE, Steenbergen C, Murphy E
American journal of physiology. Heart and circulatory physiology 2003 Dec;285(6):H2657-62
American journal of physiology. Heart and circulatory physiology 2003 Dec;285(6):H2657-62
Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat.
Kögler H, Hartmann O, Leineweber K, Nguyen van P, Schott P, Brodde OE, Hasenfuss G
Circulation research 2003 Aug 8;93(3):230-7
Circulation research 2003 Aug 8;93(3):230-7
Supramolecular calsequestrin complex.
Glover L, Quinn S, Ryan M, Pette D, Ohlendieck K
European journal of biochemistry 2002 Sep;269(18):4607-16
European journal of biochemistry 2002 Sep;269(18):4607-16
Supramolecular calsequestrin complex.
Glover L, Quinn S, Ryan M, Pette D, Ohlendieck K
European journal of biochemistry 2002 Sep;269(18):4607-16
European journal of biochemistry 2002 Sep;269(18):4607-16
Augmented expression of cardiotrophin-1 in failing human hearts is accompanied by diminished glycoprotein 130 receptor protein abundance.
Zolk O, Ng LL, O'Brien RJ, Weyand M, Eschenhagen T
Circulation 2002 Sep 17;106(12):1442-6
Circulation 2002 Sep 17;106(12):1442-6
A novel and rapid approach to isolating functional ryanodine receptors.
West DJ, Smith EC, Williams AJ
Biochemical and biophysical research communications 2002 Jun 7;294(2):402-7
Biochemical and biophysical research communications 2002 Jun 7;294(2):402-7
Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality.
Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GW 2nd, Kranias EG
Circulation 2001 Feb 13;103(6):889-96
Circulation 2001 Feb 13;103(6):889-96
Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality.
Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GW 2nd, Kranias EG
Circulation 2001 Feb 13;103(6):889-96
Circulation 2001 Feb 13;103(6):889-96
Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium.
Kobayashi YM, Jones LR
The Journal of biological chemistry 1999 Oct 1;274(40):28660-8
The Journal of biological chemistry 1999 Oct 1;274(40):28660-8
Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane.
Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR
The Journal of biological chemistry 1997 Sep 12;272(37):23389-97
The Journal of biological chemistry 1997 Sep 12;272(37):23389-97
Residues 2-25 of phospholamban are insufficient to inhibit Ca2+ transport ATPase of cardiac sarcoplasmic reticulum.
Jones LR, Field LJ
The Journal of biological chemistry 1993 Jun 5;268(16):11486-8
The Journal of biological chemistry 1993 Jun 5;268(16):11486-8
Developmental changes in cardiac sarcoplasmic reticulum in sheep.
Mahony L, Jones LR
The Journal of biological chemistry 1986 Nov 15;261(32):15257-65
The Journal of biological chemistry 1986 Nov 15;261(32):15257-65
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
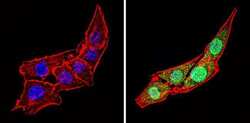
- Experimental details
- Immunofluorescent analysis of Calsequestrin (green) showing staining in the cytoplasm and nucleus of C2C12 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Calsequestrin polyclonal antibody (Product # PA1-913) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
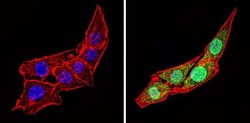
- Experimental details
- Immunofluorescent analysis of Calsequestrin (green) showing staining in the cytoplasm and nucleus of C2C12 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Calsequestrin polyclonal antibody (Product # PA1-913) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
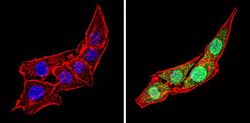
- Experimental details
- Immunofluorescent analysis of Calsequestrin (green) showing staining in the cytoplasm and nucleus of C2C12 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Calsequestrin polyclonal antibody (Product # PA1-913) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4 ºC in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a fluorescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
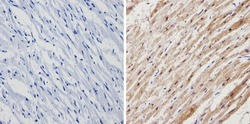
- Experimental details
- Immunohistochemistry analysis of Calsequestrin showing positive staining in the cytoplasm of paraffin-treated Human heart tissue (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Calsequestrin polyclonal antibody (Product # PA1-913) diluted by 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
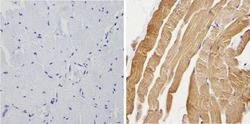
- Experimental details
- Immunohistochemistry analysis of Calsequestrin showing positive staining in the cytoplasm of paraffin-treated Human skeletal muscle (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Calsequestrin polyclonal antibody (Product # PA1-913) diluted by 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
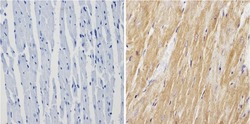
- Experimental details
- Immunohistochemistry analysis of Calsequestrin showing positive staining in the cytoplasm of paraffin-treated Mouse heart tissue (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval method was performed using 10mM sodium citrate (pH 6.0) microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Calsequestrin polyclonal antibody (Product # PA1-913) diluted by 3% BSA-PBS at a dilution of 1:200 overnight at 4°C in a humidified chamber. Tissues were washed extensively PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
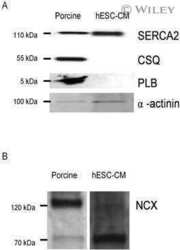
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
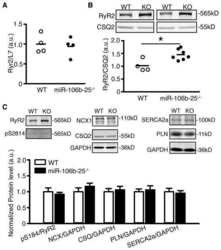
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
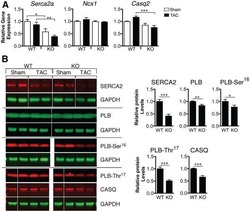
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
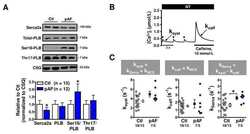
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
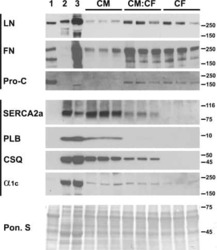
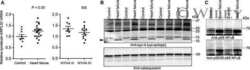
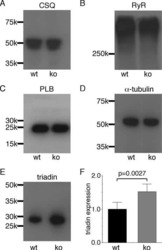
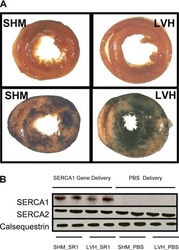
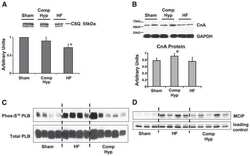
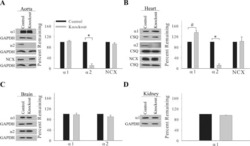
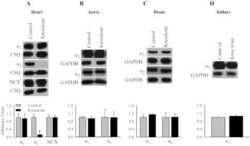
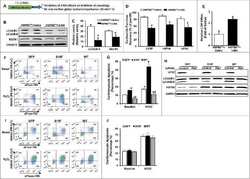
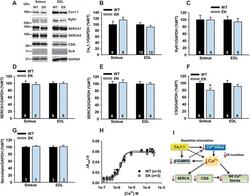
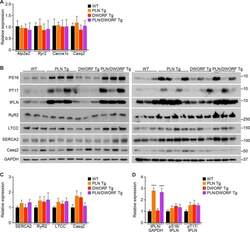
- Figure 3 Ca 2+ handling proteins. (A) Representative western blot images of Ca V 1.1, RyR1, SERCA1, SERCA2, calsequestrin (1 and 2), and sarcolipin. To allow the use of data in multiple western blots each band within a single western blot was normalized to GAPDH for that sample as a loading control and then normalized to the average WT values for that particular gel to give %WT. (B) Analysis of muscle levels of Ca V 1.1 normalized to GAPDH. (C) Analysis of muscle levels of RyR1 normalized to GAPDH. (D) Analysis of muscle levels of SERCA1 normalized to GAPDH. (E) Analysis of muscle levels of SERCA2 normalized to GAPDH. (F) Analysis of muscle levels of CSQ normalized to GAPDH. (G) Analysis of sarcolipin normalized to GAPDH. (H) SERCA activity as a function of Ca 2+ concentration. (I) Scheme of changes in Ca 2+ handling proteins. Values are shown as mean +- SEM. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2--figure supplement 2. Analysis of RNA and protein expression levels of the major cardiac Ca 2+ -handling proteins in PLN/DWORF Tg mice. ( A ) RNA levels of the indicated genes as quantified by qRT-PCR in 16-week-old heart tissue. Atp2a2 , SERCA2a; Ryr2 , ryanodine receptor 2; Cacna1c, alpha1C-subunit of the L-type Ca 2+ channel; Casq2 , calsequestrin 2. Data are normalized to 18S and presented as expression level relative to WT, mean +-SD for n = 10 mice per genotype. ( B ) Representative immunoblots of cardiac homogenates from mice with the indicated genotypes. PS16, phospho-serine 16 on PLN; PT17, phospho-threonine 17 on PLN; tPLN, total phospholamban; RyR2, ryanodine receptor 2; LTCC, L-type Ca 2+ channel (alpha1C-subunit); Casq2, calsequestrin 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. ( C ) Western blots for n = 9-10 mice of each genotype were quantified using ImageJ and data are normalized to GAPDH and expressed as mean +-SD relative to WT. ( D ) Quantification of total phospholamban and its phosphorylation status as assessed by western blot ( B ) and expressed as relative to WT. Western blots were quantified with ImageJ software. Phosphorylation blots (PS16 and PT17) were normalized to total PLN (tPLN). Total PLN was normalized to GAPDH. Data are expressed as mean +-SD for n = 9-10 mice per genotype. Statistical comparisons between groups were evaluated by Student's t-test. p-value *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
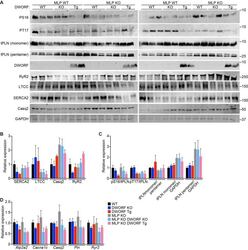
- Experimental details
- Figure 6--figure supplement 1. Expression and post-translational modifications of Ca 2+ -handling proteins. ( A ) Immunoblots of cardiac homogenates from mice with the indicated genotypes. ( B ) Immunoblots were quantified using ImageJ and normalized to GAPDH. LTCC, L-type Ca 2+ channel (alpha1C-subunit); Casq2, calsequestrin 2; RyR2, ryanodine receptor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. ( C ) Quantification of the phosphorylation status and oligomerization of phospholamban as assessed by western blot (panel A ) and expressed as relative to WT. Western blots were quantified with ImageJ software. Phosphorylation blots (PS16 and PT17) were normalized to total PLN (tPLN). Total PLN was normalized to GAPDH. tPLN, total phospholamban; PS16, phospho-serine 16 on PLN; PT17, phospho-threonine 17 on PLN. Data are expressed as mean +-SD for n = 4-6 mice per genotype. ( D ) RNA levels of the indicated genes as quantified by qRT-PCR in 8-week-old heart tissue. Atp2a2 , SERCA2a; Cacna1c, alpha1C-subunit of the L-type Ca 2+ channel; Casq2 , calsequestrin 2; Pln , phosholamban; Ryr2 , ryanodine receptor 2. Data are normalized to 18S values and presented as expression level relative to WT, mean +-SD for n = 4-5 mice per genotype.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
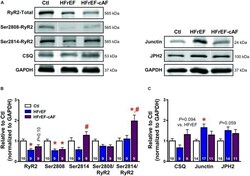
- Experimental details
- FIGURE 8 Molecular determinants of ryanodine receptor type-2 (RyR2) dysfunction. (A) Representative Western blot examples of total RyR2, Ser2808-, and Ser2814-phosphorylated RyR2 and calsequestrin (CSQ; left), as well as junctin and junctophilin-2 (JPH2; right) protein expression levels. GAPDH was used as loading control. (B) Quantification of total RyR2, Ser2808-, and Ser2814-phosphorylated RyR2, Ser2808/total RyR2, and Ser2814/total RyR2 protein expression levels in Ctl (white bars), HFrEF (blue bars) and HFrEF-cAF (red/blue-striped bars) patients. (C) Protein expression levels of the RyR2-interacting proteins CSQ, junctin, and JPH2 in Ctl, HFrEF and HFrEF-cAF patients. Data are shown relative to Ctl. Numbers in bars indicate number of patients. * indicates P < 0.05 vs. Ctl, # indicates P < 0.05 vs. HFrEF.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
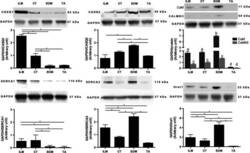
- Experimental details
- Figure 2 Protein levels of sarcoplasmic reticulum Ca 2+ -handling proteins in intrinsic laryngeal muscles (ILM), cricothyroid (CT) muscle, extraocular muscles (EOM), and tibialis anterior (TA) muscle. Western blot analysis showing relative abundance of indicated proteins: calsequestrins 1 and 2 (CASQ1 and CASQ2), Sercas 1 and 2 (SERCA1 and SERCA2), calmodulin (CaM), calmodulin kinase II (CaMKII), and Orai1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for protein loading, Western blot transfer and nonspecific changes in protein levels. The molecular weight, expressed in kDa, for each protein is indicated. Quantifications from three independent muscle samples, each sample containing muscles pooled from three or four different rats. Asterisks and different letter combination indicate statistical significance (* P < 0.05 and ab, ac, ad, bc, or bd P < 0.05, respectively). In ILM, CASQ 1 was more abundant than CASQ2 compared with TA. SERCA1 was less than SERCA2 in ILM and higher compared with TA. CT and the other ILM showed similar levels of the proteins studied.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
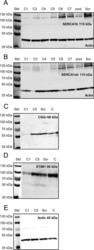
- Experimental details
- Fig 1 Effect of SERCA1b shRNA on the expression pattern of proteins involved in Ca 2+ -homeostasis. Examination of decreased SERCA1b expression in C2C12 myotubes. ( A-B ) Protein expression of SERCA was detected by Western-blot analysis to prove the efficiency of SERCA1b-specific shRNA in multinucleated myotubes. Different stably transfected clones, pool of the clones and scrambled shRNA transfected cells were compared. The 115 kDa isoforms were detected either by SERCA1b specific antibody corresponding to the terminal octamer of the protein or by an antibody recognizing both SERCA1a and b isoforms. Actin was used as a control. ( C ) Western blot analysis to detect the SR calcium-binding protein (calsequestrin) expression in selected cloneC1, C5, scrambled shRNA transfected, and parental cells. ( D ) Western-blot analysis showing the protein level of the key molecule of SOCE (STIM1). Total protein samples were used (30 mug in each lane) to examine the protein expression level. For preparing protein samples cultures were harvested on the 5 th day of differentiation in each cases. Representative data of 3 independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
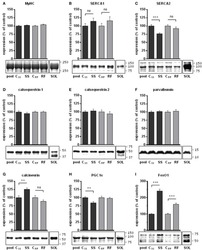
- Experimental details
- Figure 3 Expression levels of selected proteins in gastrocnemius extracts from semistarved and refed rats and their respective controls were determined by Western blots . (A) Amount of myosin heavy chains (MyHC) were determined by Coomassie staining in 3 independents experiments. MyHC were similarly abundant in all 4 groups and were thus validated as internal controls for the quantification of the other muscle markers under study. Selected proteins that are well known markers of Ca 2+ handling in slow-twitch or fast-twitch fibers were analyzed: (B) SERCA1, (C) SERCA2, (D) calsequestrin 1, (E) calsequestrin 2, and (F) parvalbumin. The abundance of transcription factors that control the development of slow-twitch muscles either positively or negatively were also determined: (G) calcineurin, (H) PGC1-alpha (the most intense band at the expected size for the full-length protein was analyzed; the smaller molecular weight bands likely to be degradation products were excluded), and (I) FoxO1 (all bands, likely representing native, methylated, and acetylated forms, were quantified). The signals (B-I) were corrected for their MyHC content and normalized to the signal of a pool sample (a mixture of aliquots of all extracts) loaded on the gels for the purpose of intra-gel and inter-gel comparison. The signals from an extract of soleus muscle (a slow-twitch muscle), referred to as SOL, are shown for comparison. They were acquired under the same exposure condition as the experimental grou
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
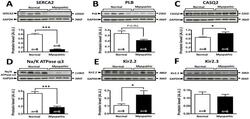
- Experimental details
- Figure 5 Comparison of protein expression in LV sample from control and cardiomyopathic patients. A: Top; representative SERCA2 and respective GAPDH stainings obtained in normal and cardiomyopathic LV samples. Bottom; Mean+-SEM, *** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
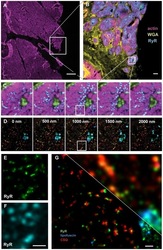
- Experimental details
- Figure 3 Correlative confocal and super-resolution imaging of a human cardiac tissue section. The section was ~10 um thick and was labeled with phalloidin for f-Actin (Alexa 488), WGA for the cell membrane and extracellular matrix (Alexa 594), along with antibodies for the ryanodine receptor (RyR, Alexa 680) and calsequestrin (CSQ, Alexa 750). In addition to the applied labelling, a strong endogenous fluorescence signal from lipofuscin was recorded. The shorter wavelength labels (Actin, WGA, and RyR) were imaged on a confocal microscope, and the sample then taken to the localisation microscope where super-resolution imaging of the longer wavelength labels (RyR, lipofuscin, CSQ) was performed. Panel A shows an overview of the cellular structure across a large tissue area that is indicated by the actin labeling (largely muscle cell contractile protein). Scale bar 100 um. Panel B is a projection of a confocal stack taken of the region indicated in A. Scale bar 10 um. Panel C shows a confocal stack of a small detail area from B and panel D shows an optically sectioned super-resolution stack, within the region covered by the confocal stack in C. Panels E & F compare corresponding confocal (F) and super-resolution (E) images both using the RyR-Alexa 647 signal. Note the good correlation between the data and the improvement in resolution in E. Scale bar 1 um. G : 3-colour super-resolution image of a small area in the tissue sample, note the improved resolution as compared to the con
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
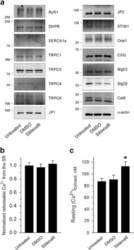
- Experimental details
- Figure 5 Expression level of skeletal muscle proteins, the amount of releasable Ca 2+ from the SR to the cytosol, and the resting cytosolic Ca 2+ level in primary skeletal myotubes. ( a ) The immunoblot analysis of proteins mediating Ca 2+ movements and handling in skeletal muscle was conducted using the lysate of myotubes treated with sildenafil. Fifteen proteins were examined, and no expression levels were changed by sildenafil (the expression level of proteins is presented as bar graphs in Supplementary Figure 5 ). alpha-Actin was used as a loading control. At least three independent experiments per protein were conducted and a representative result is presented. JP, junctophilin; CSQ, calsequestrin; Mg, mitsugumin; CaM, calmodulin. The amount of releasable Ca 2+ from the SR to the cytosol in response to TG (2.5 mu M ) ( b ) or resting cytosolic Ca 2+ level ( c ) was examined in myotubes treated with sildenafil, and histograms are shown for the normalized peak amplitude or area under the peak to the mean value of those from untreated controls as described in the 'Materials and methods' section. TG was applied to myotubes in the absence of extracellular Ca 2+ to avoid extracellular Ca 2+ entry. The results are presented as the means+-s.e. for the number of experiments presented in the parentheses of Table 2 . *, and the significant difference was compared with the untreated control ( P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
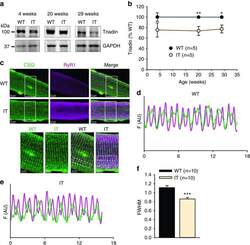
- Experimental details
- Figure 3 Effect of the IT mutation on triadin and CSQ. ( a ) Representative western blots of triadin levels in soleus muscle from WT and IT mice at different ages. ( b ) Analysis of triadin levels. ( c ) RyR1 and CSQ localization in FDB fibres by immunocytochemistry. Magenta: RyR1, Green: CSQ. ( d , e ) Line scans of CSQ and RyR1 immunostaining in FDB fibres of IT ( e ) and WT ( d ) mice. ( f ) Analysis of the fluorescence profile of CSQ in WT and IT fibres, FWHM is full width at half maximum. Data are shown as mean+-s.e.m. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
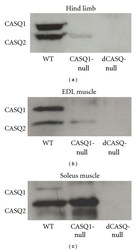
- Experimental details
- Figure 1 dCASQ- mice did not express any of the two CASQ isoforms. Western blot analysis of total homogenates prepared from hind limb (a), EDL, and Soleus (b and c) muscles showed that (i) in CASQ1- muscles CASQ1 was missing, whereas CASQ2 was still present, more in Soleus (slow twitch) and less in EDL (fast twitch); (ii) in dCASQ- muscles both isoforms were absent.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
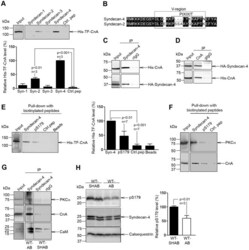
- Experimental details
- Figure 4 Association of syndecan-4 with calcineurin A (CnA). (A) Pull-down experiments with biotinylated peptides covering cytoplasmic domains of syndecan-1-4 were performed using recombinant His-trigger factor (TF)-CnA, and precipitates immunoblotted for presence of His-TF-CnA (n = 3). (B) Alignment of syndecan-4 and syndecan-2. Black boxes indicate similar amino acids within consensus region. Lysates from HEK-293 cells co-transfected with syndecan-4 and CnA subjected to immunoprecipitation with (C) anti-syndecan-4 (n = 3) or (D) anti-CnA (n = 3) were immunoblotted with anti-CnA or anti-syndecan-4, respectively. Pull-down experiments with biotinylated peptides covering non-phosphorylated or phosphorylated cytoplasmic part of syndecan-4 were performed using recombinant His-TF-CnA (E) or left ventricular (LV) tissue before immunoblotted for presence of endogenous CnA (n = 3) and PKC-alpha (n = 2) (F). (G) LV tissue extracts from WT-SHAB and WT-AB were immunoprecipitated with anti-syndecan-4 or control IgG and lysates and immunoprecipitates immunoblotted for presence of PKC-alpha, CnA and calmodulin (CaM, n = 2). (H) Relative pS179-syndecan-4/total syndecan-4 levels in aorta-banded (AB) versus sham-operated (SHAB) WT LV tissue (n = 3). Calsequestrin was used for loading control. Values are mean +- s.e.m.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
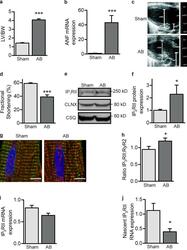
- Experimental details
- Figure 1. IP 3 RII is up-regulated post-transcriptionally during hypertrophy in cardiac myocytes. (a) Quantification of the ratio of left ventricular weight to body weight in sham and AB adult rats. (b) Analysis of ANF mRNA expression by qPCR in sham and AB left ventricular samples. (c) Representative echocardiography of sham and AB rats. Dimensions indicate left ventricular diastolic dimension (LVDd) and posterior wall thickness (PWd), indices of ventricular function and hypertrophy, respectively. (d) Fractional shortening quantification (%) from echocardiographic analysis of eight sham and six AB rats. (e) Immunoblot for IP 3 RII in the membrane protein fraction of sham and AB ventricular lysates. Calnexin (CLNX) and calsequestrin (CSQ) are shown as loading controls. (f) Quantification of immunoblots as in e; n = 5. (g) Immunofluorescence image of ventricular myocytes isolated from sham and AB rats. IP 3 RII is shown in red, RyR2 in green, and DAPI in blue. Bar, 5.8 um. (h) Ratio of IP 3 RII to RyR2 quantified from immunofluorescence images as in g. For the sham condition, 16 cells from 2 independent preparations were analyzed. For the AB condition, 26 cells from 2 independent preparations were analyzed. (i) IP 3 RII mRNA expression measured by qPCR in sham/AB samples. (j) Nascent IP 3 RII RNA expression measured by qPCR in the sham/AB samples. Bar graphs are mean +- SEM from at least three independent experiments. *, P < 0.05; ***, P < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 Validation of a few selected 2D DIGE proteins. (A), representative immunoblots of SLMAP, NDRG2, ERP29, and CRYAB expression levels in all experimental groups. Western blot of calsequestrin (CSQ) was used as a control of protein loading, and to normalize the densitometric data of each protein expression. (B-E), respective histograms of levels of SLMAP, NDRG2, ERP29, and CRYAB proteins (are expressed as fold) increases in TAC mouse groups (C and H) relative to their respective shams (S), in the FKBP12.6 overexpressing (K) group compared with wild-type mice (W) of both genders (M or F). Data are mean +- sem. * P < 0.05, TAC versus sham; $ P < 0.05, C versus. H; # P < 0.05, female versus male.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
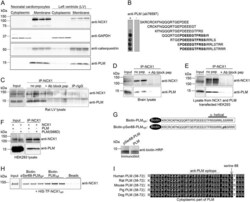
- Experimental details
- Figure 1 Confirmation of a direct PLM-NCX1 interaction ( A ) NCX1 and PLM were analysed in cytoplasmic and membrane fractions isolated from rat neonatal cardiomyocytes and LV using anti-NCX1 and anti-PLM antibodies. GAPDH and calsequestrin were used as controls for cytoplasmic and membrane fractions respectively. ( B ) Epitope mapping was performed by overlaying an array of immobilized overlapping 20-mer PLM peptides with anti-PLM (ab76597, left-hand panel). Amino acids in bold were relevant for anti-PLM binding. Immunoblotting without anti-PLM was used as a negative control (right-hand panel). ( C ) Rat LV, ( D ) brain or ( E and F ) lysate from HEK-293 cells co-transfected with NCX1 and PLM or PLM(S68D) was subjected to immunoprecipitation using anti-NCX1. Immunoprecipitates and lysate was immunoblotted with anti-NCX1 and anti-PLM antibodies. A specific anti-NCX1 blocking peptide and non-relevant rabbit IgG were used as negative controls. ( G ) Schematic presentation of biotinylated peptides covering PLM cyt and pSer 68 -PLM cyt (upper panel). The alpha-helical region is indicated. Immunoblotting analysis of the two biotinylated peptides using HRP-conjugated anti-biotin is shown in the lower panel. ( H ) Pull-down assay with biotin-PLM cyt and biotin-pSer 68 -PLM cyt against recombinant His-TF-NCX1 cyt (containing the cytoplasmic part of NCX1) using monoclonal anti-biotin-conjugated beads. Pull-down of NCX1 was analysed by immunoblotting using anti-NCX1. ( I ) Alignment of
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
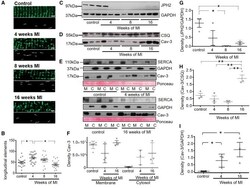
- Experimental details
- Figure 3 Transiently increased occurrence of longitudinal elements in the TAT system is accompanied by a persistent decrease in JPH2 post-MI and dissociation of Cav-3 into the cytosol. The occurrence of longitudinal elements of the TAT system transiently rises while JPH2 levels decrease and remain decreased from the earliest time point post-MI and Cav-3 levels increase and dissociate from the membrane 16-week post-MI. (A) Top panels: representative 40 x 10 um frames of the confocal images of cardiomyocytes at different time points (Scale bar equals 2 mum); Bottom panels: axial elements extracted mathematically from frames above as explained in 'Methods' section; (B) Number of longitudinal tubules; * P < 0.05 as calculated with mixed ANOVA followed by Wald chi 2 -test. Sample number N/n = 4/20 each; (C) Representative immunoblots for JPH2 and (D) of Cav-3 as well as the house keeping gene products GAPDH and CSQ, each sample was analysed in triplicates from 3 different animals (control = age matched animals); (E) Representative immunoblots showing dissociation of Cav-3 protein from membrane to cytosol at 4- and 16-week post-MI. SERCA2a was used as membrane fraction marker and GAPDH as cytosolic fraction marker. Ponceau staining was performed as overall loading control. (F) and (G) Density of respective JPH2 bands in (C) normalized to corresponding GAPDH bands, n = 3 each, and of Cav-3 bands in (D) normalized to CSQ bands. (H) Density of Cav-3 in cytosolic fractions of
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
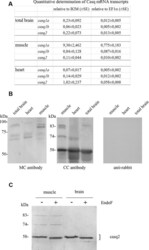
- Experimental details
- Figure 1 Casq mRNA in selected tissues from adult zebrafish (A) . The levels of RNA expression are relative to two different housekeeping genes, beta-2-microglobulin (B2M) and elongation factor1-alpha (EF1alpha). Mean values and standard error were determined from triplicate runs of the qPCR assay in three different tissue preparations. (B) . Anti-Casq antibodies reactivity on total homogenates (40 mug per lane) obtained from pooled (N = 3) skeletal muscle, heart, and brain of adult zebrafish. In the control lanes, (anti-rabbit) blot incubation was performed in the presence of anti-rabbit conjugate with alkaline phosphatase only. Molecular mass markers (in kDa) are indicated. (C) In vitro deglycosylation of Casqs. Immunodetection of Casq by CC antibody in membrane-enriched fraction P4 (see ""Materials and Methods"" section) after endoglycosidase F digestion, with 10 mug loaded per lane.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
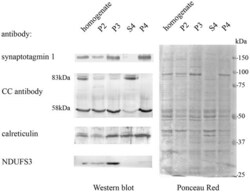
- Experimental details
- Figure 2 Adult brain subcellular fractionation: immunochemical profile. Immunoblot analysis of brain subcellular fractions. Equal protein amounts (40 mug) from each fraction were analyzed with CC antibodies to recognize Casqs and with antibodies specific for synaptotagmin1 (SYT1), calreticulin, NDUFS3 (as described in ""Materials and Methods"" section). Raw data derived from densitometric analysis are presented in Supplementary Table S1 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 5 Immunofluorescence staining of sagittal brain sections area restricted to the cerebellum and the optic tectum (rostral to the right) decorated with CC (A,C-E) and MC (F,H-K) antibodies. (B,G) Images collected from control sections (see ""Results"" section) and acquired with the same conditions as in (A) and (F) , respectively. (A,B,F,G) Epi-fluorescence signals. (C-E,H-K) Images obtained by single optic section of confocal analysis. The box in (C) indicates the area shown at higher magnification in (E) . (D) PCL in a different region and focal plane. Arrows: axons of Purkinje cells. The abbreviations are the same as in Figure 4 . (A,C,F,H,I) Bar: 100 mum. (E) Bar: 25 mum. (D,J,K) Bar: 7.5 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
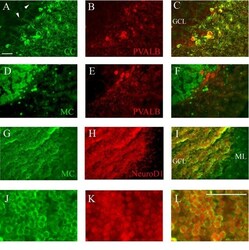
- Experimental details
- Figure 6 Co-staining of cerebellum with Casq and neuron-specific markers. (A-C) Epi-fluoresence signal obtained by double immunofluorescence with CC antibody (green) and anti-parvalbunin antibodies (red) in the cerebellum area. (C) A merge of the two images. Arrowheads indicate the axons of Purkinje cells. (D-F) Epi-fluoresence images obtained by double-staining with MC antibody (green) and anti-parvalbunin antibodies (red). (D) A merge of the two images. (G-I) Confocal images obtained by double-staining with MC antibody (green) and anti-NeuroD1 antibody (red). (J-L) Higher magnification of granule cells in the central area of the cerebellum double-labeled with MC (green) and anti-NeuroD1 antibody (red). Bar: 25 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
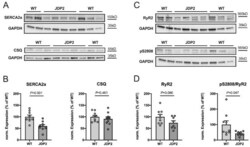
- Experimental details
- Figure 5 Expression and phosphorylation of sarcoplasmic reticulum (SR) calcium-handling proteins in atria from JDP2-overexpressing mice. Original Western blots ( A , C ) and summarized data ( B , D ) from 8-9 atria isolated from mice overexpressing JDP2 for 5 weeks vs. WT mice ( p -values as indicated). Expression of SERCA2a, CSQ and RyR2 was analyzed, as well as phosphorylation of RyR2 at S2808. GAPDH was used as loading control for normalization. Note that the GAPDH signals shown in ( A ) for SERCA2a and in ( C ) for pS2808 are identical, as the proteins were derived from the same Western blot.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
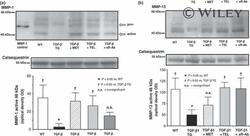
- Experimental details
- 2 (a) Representative immunoblot of the collagenase matrix metalloproteinase-1 at 57/52 kDa (proMMP-1, latent) and active MMP-1 at 46 kDa. MMP-1 control = MMP-1 protein control (Sigma-Aldrich, Deisenhofen, Germany), WT = wild type, TGF-beta1- TG = Alb/TGF-beta1 (Cys223,225Ser) transgenic mice at 8 weeks of age, MET = Metoprolol, TEL = Telmisartan, TGF-beta1-sR-Ab = soluble TGF-beta1 receptor antibody (upper panel). Western blot data were normalized to calsequestrin as a non-regulated protein. Densitometric quantification of the active MMP-1 protein expression is shown (lower panel). Data are represented as mean +- SEM; n = 5 per group. (b) Representative immunoblot of matrix metalloproteinase-13 (MMP-13) protein expression at 48 kDa (active form). WT = wild type, TGF-beta1-TG = Alb/TGF-beta1 (Cys223,225Ser) transgenic mice at 8 weeks of age, MET = Metoprolol, TEL = Telmisartan, TGF-beta1-sR-Ab = soluble TGF-beta1 receptor antibody (upper panel). Western blot data were normalized to calsequestrin as a non-regulated protein. Densitometric quantification of the active collagenase MMP-13 is shown (lower panel). Data are represented as mean +- SEM; n = 5 per group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
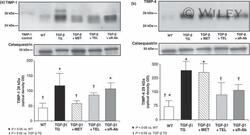
- Experimental details
- 3 (a) Representative immunoblot of tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) protein expression at 28 kDa. TIMP-1-control = TIMP-1 protein control (Sigma-Aldrich, Deisenhofen, Germany), WT = wild type, TGF-beta1-TG = Alb/TGF-beta1 (Cys223,225Ser) transgenic mice at 8 weeks of age, MET = Metoprolol, TEL = Telmisartan, TGF-beta1-sR-Ab = soluble TGF-beta1 receptor antibody (upper panel). Western blot data were normalized to calsequestrin as a non-regulated protein. Densitometric quantification of the collagenase inhibitor TIMP-1 is shown (lower panel). Data are represented as mean +- SEM; n = 5 per group. (b) Representative immunoblot of tissue inhibitor of matrix metalloproteinase-4 (TIMP-4) protein expression at 29 kDa. WT = wild type, TGF-beta1-TG = Alb/TGF-beta1 (Cys223,225Ser) transgenic mice at 8 weeks of age, MET = Metoprolol, TEL = Telmisartan, TGF-beta1-sR-Ab = soluble TGF-beta1 receptor antibody (upper panel). Western blot data were normalized to calsequestrin as a non-regulated protein. Densitometric quantification of the MMP-inhibitor TIMP-4 is shown (lower panel). Data are represented as mean +- SEM; n = 5 per group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
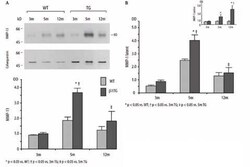
- Experimental details
- Fig. 2 A Immunoblot analysis of MMP-13 collagenase protein expression and densitometric quantification. Corresponding blot of the housekeeping protein calsequestrin is shown below. B Densitometric quantification of the collagenase pro-MMP-1 (latent) and active MMP-1 protein expression measured by immunoblotting
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
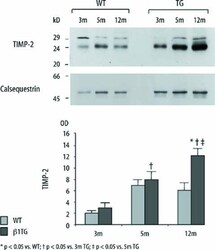
- Experimental details
- Fig. 4 Immunoblot and densitometric quantification of tissue inhibitor of matrix metalloproteinase-2 (TIMP-2; 24 kD). TIMP-2 expression continuously increased in beta1TG mice with progressive interstitial fibrosis and ventricular dysfunction. Corresponding blot of the housekeeping protein calsequestrin is shown below; n=5/group
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
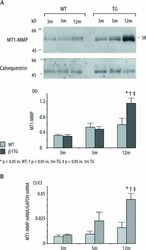
- Experimental details
- Fig. 5 A Immunoblot and densitometric quantification of membrane-type-1 MMP protein (58 kD) expression of beta1TG mice and WT. Corresponding blot of the housekeeping protein calsequestrin is shown below; n=5/group. B Quantitative real-time PCR of MT1-MMP mRNA. Ct values are presented as a ratio of MT1-MMP mRNA to the internal standard GAPDH mRNA
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
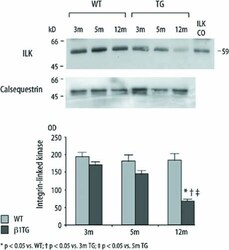
- Experimental details
- Fig. 6 Immunoblot and densitometric quantification of integrin-linked kinase (ILK) protein expression of beta1TG and age matched WT. ILK is recognized at 59 kD (ILK control) and significantly downregulated in mice with severe interstitial fibrosis and ventricular dilatation at 12 months of age. Corresponding blot of the housekeeping protein calsequestrin is shown below
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
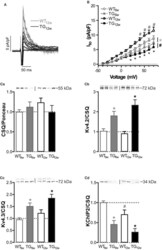
- Experimental details
- Figure 2 Transient outward K + channel expression in transgenic atrial myocytes. Experimental animals involved wild-type mice (WT) and transgenic mice (TG) expressing the repressor isoform of cyclic adenosine monophosphate response element modulator, CREM-IbDeltaC-X, and were distributed according to age in 6-week-old (WT 6w and TG 6w ) and 12-week-old (WT 12w and TG 12w ) groups. A , Representative transient outward K + current (I to ) traces recorded as -40 mV sensitive currents from atrial cardiomyocytes of WT 12w and TG 12w . For clarity, current density (expressed in picoamperes/picofarads [pA/pF]) traces from -40 mV with +20 mV increments are displayed. B , Mean+-SE of current-voltage plots of the peak current density (I to ). For WT 6w , n/N are 41/15, for TG 6w n/N are 22/14, for WT 12w n/N are 35/19, and for TG 12w n/N are 20/16. Ca through d , Representative immunoblots and quantification of protein expression of calsequestrin (CSQ; Ca ) normalized to Ponceau and of K + voltage-gated channel subfamily D member 2 (Kv4.2, Cb ) and 3 (Kv4.3, Cc ), and K + channel interacting protein 2 (KChIP2) ( Cd ) normalized to CSQ. Data show mean+-SE of N=7 mice per group, except for Kv4.3, where N=6 as the result of an undetectable band in TG 12w . * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
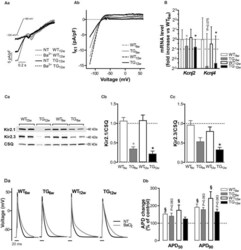
- Experimental details
- Figure 3 Inward rectifier K + current I K1 expression and function in transgenic atrial myocytes. Experimental animals involved wild-type mice (WT) and transgenic mice (TG) expressing the repressor isoform of cyclic adenosine monophosphate response element modulator, CREM-IbDeltaC-X, and were distributed according to age in 6-week-old (WT 6w and TG 6w ) and 12-week-old (WT 12w and TG 12w ) groups. A , Representative traces of inward rectifiers K + channel (I K1 ) recorded in WT 12w and TG 12w myocytes before and after application of 10 mumol/L BaCl 2 using a ramp protocol (inset, scale bar 0.2 seconds). The traces show current densitiy (expressed in picoamperes/picofarads [pA/pF]) over time. ( Aa ). Current-voltage plots of I K1 averaged from traces of all cells. For WT 6w , n/N are 8/5, for TG 6w n/N are 10/6, for WT 12w n/N are 13/7, and for TG 12w n/N are 9/6 ( Ab ). B , Data show mean+-SE of relative mRNA levels of atrial K + voltage-gated channel subfamily J member 2 ( Kcnj2 ) and 4 ( Kcnj4 ) normalized to WT 6w vs Hprt1 (hypoxanthine phosphoribosyltransferase 1) as the housekeeping gene; N=8 per group. Y-axes scale is Log2. Ca through c , Representative immunoblots ( Ca ) and quantification of K + inwardly rectifying channel subunits Kir2.1 ( Cb ) and Kir2.3 ( Cc ) protein levels normalized to calsequestrin (CSQ). Data show mean+-SE of 7 mice per group. Da through b , Representative action potentials recorded before (normal Tyrode [NT] as control) and after application
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
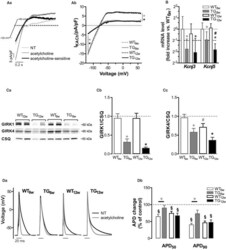
- Experimental details
- Figure 4 Acetylcholine-activated K + current (I KACh ) expression and function in transgenic atrial myocytes. Experimental animals involved wild-type mice (WT) and transgenic mice (TG) expressing the repressor isoform of cyclic adenosine monophosphate response element modulator, CREM-IbDeltaC-X, and were distributed according to age in 6-week-old (WT 6w and TG 6w ) and 12-week-old (WT 12w and TG 12w ) groups. A , Representative traces recorded before and after application of 10 mumol/L acetylcholine using the ramp protocol (inset, scale bar 0.2 seconds) in 1 isolated WT 12w atrial cardiomyocyte. The acetylcholine-sensitive current derived by subtraction is I KACh . The traces show current densitiy (expressed in picoamperes/picofarads [pA/pF]) over time. ( Aa ). Current-voltage plots of I KACh averaged from traces of all cells. n/N for WT 6w are 12/6, for TG 6w are 13/7, for WT 12w are 12/6, and for TG 12w are 10/6 ( Ab ). B , Relative mRNA levels of K + voltage-gated channel subfamily J members 3 ( Kcnj3 ) and 5 ( Kcnj5 ) measured in atrial tissue, with Hprt1 (hypoxanthine phosphoribosyltransferase 1) as the housekeeping gene. Data were normalized to WT 6w . Y-axes scale is Log2. Data show mean+-SE of 8 per group. Ca through c , Representative immunoblots ( Ca ) and quantification of G protein-coupled inwardly rectifying K + channel alpha subunits 1 (GIRK1, Cb ) and 4 (GIRK4, Cc ) protein levels normalized to calsequestrin (CSQ). Data show mean+-SE of N=7 mice per group. Da t
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
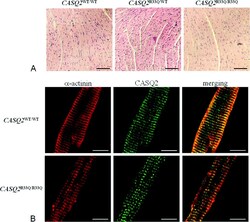
- Experimental details
- Figure 2. A, Exemplificative ventricular sections (hematoxylin-eosin stain) of CASQ2 WT/WT , CASQ2 R33Q/WT , and CASQ2 R33Q/R33Q (100x). Bars&equals10 &mgrm. B, Ventricular cardiomyocytes from CASQ2 WT/WT and CASQ2 R33Q/R33Q immunostained with rabbit anti-CASQ and mouse anti-alpha-actinin antibodies. Bars&equals20 &mgrm.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
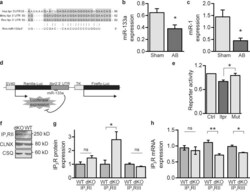
- Experimental details
- Figure 2. miR-133a regulates IP 3 RII expression. (a) Conservation of the miR-133a seed sequence between the human, mouse, and rat Itpr2 3'UTR. (b) miR-133a expression in sham and AB samples measured by qPCR. (c) miR-1 expression in sham and AB samples. (d) Schematic representation of the luciferase reporter construct used to measure miR-133a repressive activity at the Itpr2 3'UTR. (e) Luciferase activity in HEK293 cells transfected with miR-133a and either the Itpr2 3'UTR reporter or mut- Itpr2 3'UTR reporter. (f) Immunoblot for IP 3 RII in left ventricular samples from wild-type (WT) or miR-133a-1, miR-133a-2 double knockout mice (dKO). (g) Quantification of immunoblots for IP 3 RI/II/III from WT/dKO samples as in f; n = 6. (h) IP 3 RI/II/III mRNA expression in samples as in f. Bar graphs represent the mean +- SEM from at least three independent experiments. *, P < 0.05; **, P < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
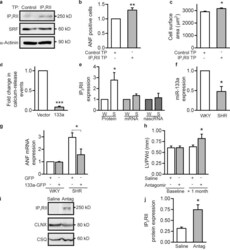
- Experimental details
- Figure 4. Antagonism of miR-133a-mediated regulation of IP 3 RII induces the hypertrophic response. (a) Immunoblot for IP 3 RII and SRF in NRVMs transfected with control or IP 3 RII-specific target protector (TP) oligos. alpha-Actinin is shown as a loading control. (b) ANF protein expression in NRVMs transfected as in a. (c) Cell surface area measurements of NRVMs transfected as in panel a. More than 500 cells were measured per condition. (d) Quantification of the fold change in the number of calcium release events elicited by IP 3 -ester in ARVMs infected with control or miR-133a adenovirus. For each condition, at least eight ARVMs were analyzed from three separate experiments. (e) IP 3 RII protein, mRNA, and nascent RNA expression in WKY and SHR myocytes measured by immunoblotting or qPCR. (f) miR-133a expression analyzed by qPCR in WKY/SHR myocytes. (g) Quantification of the hypertrophic phenotype by measurement of ANF mRNA expression in GFP/miR-133a-GFP overexpressing WKY and SHR ventricular myocytes. (h) Measurement of LVPWd by echocardiography in mice at baseline and after infusion with saline or miR-133a antagomir for 1 mo. (i) Immunoblot for IP 3 RII in left ventricular samples from mice treated with saline/antagomir for 1 mo. (j) Quantification of immunoblots as in i; n = 3. Bar graphs are mean +- SEM from at least three independent experiments. *, P < 0.05; ***, P < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
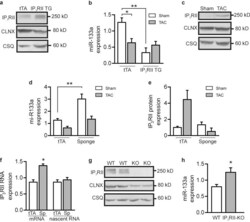
- Experimental details
- Figure 6. Manipulation of IICR in vivo alters miR-133a expression. (a) Immunoblot for IP 3 RII in left ventricular lysates from IP 3 RII transgenic mice. Tetracycline transactivator protein (tTA) mice are used as controls. (b) miR-133a expression measured by qPCR in tTA/IP 3 RII transgenic mice subjected to sham or TAC procedures. For the tTA sham/TAC and IP 3 RII-TAC conditions, three mice were analyzed. For the IP 3 RII-sham condition, two mice were analyzed. (c) Representative immunoblot for IP 3 RII in tTA ventricular lysates +- TAC. (d) qPCR analysis of miR-133a expression in ventricular lysates from tTA controls and IP 3 -sponge (sponge) expressing mice subjected to sham or TAC procedures. For the tTA sham/TAC and IP 3 -sponge-TAC conditions, three mice were analyzed. For the sponge-sham condition, two mice were analyzed. (e) Quantification of immunoblots for IP 3 RII from sham or TAC operated tTA and sponge mice. Two mice were analyzed for the tTA-sham and sponge-sham conditions. Three mice were analyzed for the tTA-TAC and sponge-TAC conditions. (f) qPCR measurement of IP 3 RII mRNA and nascent RNA expression in tTA and sponge mice. Three mice were analyzed for the tTA condition, two mice were analyzed for the sponge condition. (g) Example immunoblot of ventricular lysates from wild-type (WT) and IP 3 RII knockout (KO) mice. (h) miR-133a expression measured using qPCR in four WT and four KO mouse samples. Bar graphs show mean +- SEM. *, P < 0.05; **, P < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
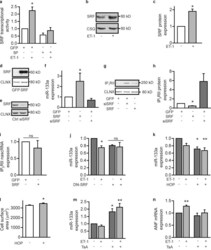
- Experimental details
- Figure 7. IICR affects miR-133a expression through modulation of SRF activity. (a) Luciferase reporter assay of SRF transcriptional activity in GFP/GFP-5P overexpressing NRVMs cultured in the presence and absence of 100 nM ET-1 for 24 h. (b) Immunoblot for SRF in control and ET-1-treated NRVM lysates. (c) Quantification of immunoblots as in b; n = 6. (d) Immunoblot for SRF in control or SRF-overexpressing NRVMs. (e) Immunoblot for SRF in control or siSRF-transfected NRVMs. (f) qPCR analysis of miR-133a expression in GFP control, SRF overexpressing, or SRF knockdown NRVMs. (g) Immunoblot to show IP 3 RII expression in GFP-control, SRF-overexpressing, and SRF knockdown NRVMs. (h) Quantification of immunoblots as in g; n = 4. (i) IP 3 RII nascent RNA expression in SRF-overexpressing NRVMs measured using qPCR. (j) qPCR analysis of miR-133a expression in control and DN-SRF-overexpressing NRVMs treated with ET-1. (k) miR-133a expression in control or HOP-overexpressing NRVMs cultured in the presence and absence of 100 nM ET-1 for 24 h. (l) Cell surface area quantification in control/HOP-overexpressing NRVMs. More than 300 cells taken from 4 separate experiments were measured per condition. (m) qPCR analysis of miR-133a expression in NRVMs treated with ET-1 in the presence and absence of 0.4 uM trichostatin A (TsA). (n) qPCR analysis of ANF expression in NRVMs treated as in k. Data are mean +- SEM from at least three independent experiments.*, P < 0.05; **, P < 0.01.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
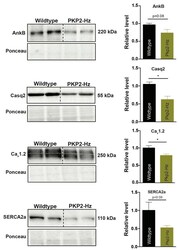
- Experimental details
- Figure 1 Remodeling of proteins involved in calcium signaling pathways in the PKP2-Hz mouse. Representative western blots (left) and average densitometry (right; n = 5 for all groups) of ankyrinB (AnkB), calsequestrin-2 (Casq2), Ca v 1.2, and SERCA2a, measured from wildtype and PKP2-Hz ventricular lysates of three-month-old mice. Left upper panels represent western blots, bottom panels according to ponceau staining used for quantification (mean +- SEM, * p < 0.05).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
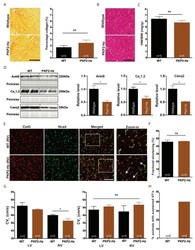
- Experimental details
- Figure 3 Pro-arrhythmic cardiac remodeling in PKP2-Hz mice exposed to exercise training. ( A ) Representative pictures of Picrosirius red staining (left panel) in the inner myocardium of wildtype and PKP2-Hz murine heart sections, of mice exposed to exercise training; scale bar, 500 um. Quantification of collagen abundance (right panel) in overview slides of whole heart sections (WT; n = 5 and PKP2-Hz; n = 5, mean +- SEM). ( B ) Hematoxylin and eosin staining of wildtype and PKP2-Hz heart sections, of mice exposed to exercise; scale bar, 100 um. ( C ) Quantification of heart weight (HW) to body weight (BW) ratio in both groups (WT; n = 5 and PKP2-Hz; n = 5, mean +- SEM). ( D ) Representative western blots (left panel) and average densitometry (right panels, n = 5 for all groups) of AnkyrinB (ANKB), Ca v 1.2, and calsequestrin-2 (Casq2) measured from wildtype (WT) and PKP2-Hz ventricular lysates of mice exposed to exercise training. Left upper panels represent western blots, bottom panels according ponceau staining used for quantification (mean +- SEM, * p < 0.05). ( E ) Immunofluorescence staining for Cx43 (red) and Ncad (green) in right ventricular heart sections of WT and PKP2-Hz mice; scale bar, 100 um. Upper right panel shows magnified shots of according merged pictures, indicated by the white boxes. Lateral Cx43 expression is more pronounced in PKP2-Hz hearts exposed to exercise training, indicated by white arrows. ( F ) Left ventricular fractional shortening examined vi
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation