Antibody data
- Antibody Data
- Antigen structure
- References [15]
- Comments [0]
- Validations
- Immunocytochemistry [6]
- Immunohistochemistry [3]
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-042 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Acetylcholinesterase Monoclonal Antibody (HR2)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA3-042 detects acetylcholinesterase (AChE) from human, mouse, rabbit, guinea pig, bovine and cat tissues. This antibody does not cross react with rat or frog AChE, and does not detect butyrylcholinesterase (BChE). MA3-042 has been successfully used in immunohistochemistry, immunoprecipitation and ELISA procedures. This antibody cannot be used in Western blot to detect AChE. Immunohistochemical staining of AChE in human brain samples with MA3-042 results in staining of nerve fibers and terminals. The MA3-042 antigen is purified human cerebellar acetylcholinesterase.
- Reactivity
- Human, Mouse, Bovine, Feline, Guinea Pig, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- HR2
- Vial size
- 200 μL
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Adventitial Cell Atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-Deficient Mice Defined by Single-Cell RNA Sequencing.
Cross-talk between microglia and neurons regulates HIV latency.
Identification of Carboxylesterase, Butyrylcholinesterase, Acetylcholinesterase, Paraoxonase, and Albumin Pseudoesterase in Guinea Pig Plasma through Nondenaturing Gel Electrophoresis.
Immunopurification of Acetylcholinesterase from Red Blood Cells for Detection of Nerve Agent Exposure.
Functional variability in butyrylcholinesterase activity regulates intrathecal cytokine and astroglial biomarker profiles in patients with Alzheimer's disease.
Lesions of cortical GABAergic interneurons and acetylcholine neurons in xeroderma pigmentosum group A.
Decrease in acetylcholinergic neurons in the pedunculopontine tegmental nucleus in a patient with Prader-Willi syndrome.
Altered levels of acetylcholinesterase in Alzheimer plasma.
Different cholinesterase inhibitor effects on CSF cholinesterases in Alzheimer patients.
Presenilin 1 interacts with acetylcholinesterase and alters its enzymatic activity and glycosylation.
Inhibition of acetylcholinesterase in CSF versus brain assessed by 11C-PMP PET in AD patients treated with galantamine.
Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer's disease following chronic donepezil treatment.
Fetal fornix transection and gestation length in sheep.
Immunocytochemical demonstration of axonal and perikaryal acetylcholinesterase in human cerebral cortex.
Monoclonal antibodies to human brain acetylcholinesterase: properties and applications.
Gu W, Ni Z, Tan YQ, Deng J, Zhang SJ, Lv ZC, Wang XJ, Chen T, Zhang Z, Hu Y, Jing ZC, Xu Q
Arteriosclerosis, thrombosis, and vascular biology 2019 Jun;39(6):1055-1071
Arteriosclerosis, thrombosis, and vascular biology 2019 Jun;39(6):1055-1071
Cross-talk between microglia and neurons regulates HIV latency.
Alvarez-Carbonell D, Ye F, Ramanath N, Garcia-Mesa Y, Knapp PE, Hauser KF, Karn J
PLoS pathogens 2019 Dec;15(12):e1008249
PLoS pathogens 2019 Dec;15(12):e1008249
Identification of Carboxylesterase, Butyrylcholinesterase, Acetylcholinesterase, Paraoxonase, and Albumin Pseudoesterase in Guinea Pig Plasma through Nondenaturing Gel Electrophoresis.
Napon G, Dafferner AJ, Saxena A, Lockridge O
Comparative medicine 2018 Oct 1;68(5):367-374
Comparative medicine 2018 Oct 1;68(5):367-374
Immunopurification of Acetylcholinesterase from Red Blood Cells for Detection of Nerve Agent Exposure.
Dafferner AJ, Schopfer LM, Xiao G, Cashman JR, Yerramalla U, Johnson RC, Blake TA, Lockridge O
Chemical research in toxicology 2017 Oct 16;30(10):1897-1910
Chemical research in toxicology 2017 Oct 16;30(10):1897-1910
Functional variability in butyrylcholinesterase activity regulates intrathecal cytokine and astroglial biomarker profiles in patients with Alzheimer's disease.
Darreh-Shori T, Vijayaraghavan S, Aeinehband S, Piehl F, Lindblom RP, Nilsson B, Ekdahl KN, Långström B, Almkvist O, Nordberg A
Neurobiology of aging 2013 Nov;34(11):2465-81
Neurobiology of aging 2013 Nov;34(11):2465-81
Lesions of cortical GABAergic interneurons and acetylcholine neurons in xeroderma pigmentosum group A.
Hayashi M, Ohto T, Shioda K, Fukatsu R
Brain & development 2012 Apr;34(4):287-92
Brain & development 2012 Apr;34(4):287-92
Decrease in acetylcholinergic neurons in the pedunculopontine tegmental nucleus in a patient with Prader-Willi syndrome.
Hayashi M, Miyata R, Tanuma N
Neuropathology : official journal of the Japanese Society of Neuropathology 2011 Jun;31(3):280-5
Neuropathology : official journal of the Japanese Society of Neuropathology 2011 Jun;31(3):280-5
Altered levels of acetylcholinesterase in Alzheimer plasma.
García-Ayllón MS, Riba-Llena I, Serra-Basante C, Alom J, Boopathy R, Sáez-Valero J
PloS one 2010 Jan 14;5(1):e8701
PloS one 2010 Jan 14;5(1):e8701
Different cholinesterase inhibitor effects on CSF cholinesterases in Alzheimer patients.
Nordberg A, Darreh-Shori T, Peskind E, Soininen H, Mousavi M, Eagle G, Lane R
Current Alzheimer research 2009 Feb;6(1):4-14
Current Alzheimer research 2009 Feb;6(1):4-14
Presenilin 1 interacts with acetylcholinesterase and alters its enzymatic activity and glycosylation.
Silveyra MX, Evin G, Montenegro MF, Vidal CJ, Martínez S, Culvenor JG, Sáez-Valero J
Molecular and cellular biology 2008 May;28(9):2908-19
Molecular and cellular biology 2008 May;28(9):2908-19
Inhibition of acetylcholinesterase in CSF versus brain assessed by 11C-PMP PET in AD patients treated with galantamine.
Darreh-Shori T, Kadir A, Almkvist O, Grut M, Wall A, Blomquist G, Eriksson B, Långström B, Nordberg A
Neurobiology of aging 2008 Feb;29(2):168-84
Neurobiology of aging 2008 Feb;29(2):168-84
Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer's disease following chronic donepezil treatment.
Darreh-Shori T, Meurling L, Pettersson T, Hugosson K, Hellström-Lindahl E, Andreasen N, Minthon L, Nordberg A
Journal of neural transmission (Vienna, Austria : 1996) 2006 Nov;113(11):1791-801
Journal of neural transmission (Vienna, Austria : 1996) 2006 Nov;113(11):1791-801
Fetal fornix transection and gestation length in sheep.
McDonald TJ, Li C, Vincent SE, Nijland MJ
Experimental neurology 2006 Aug;200(2):532-7
Experimental neurology 2006 Aug;200(2):532-7
Immunocytochemical demonstration of axonal and perikaryal acetylcholinesterase in human cerebral cortex.
Mesulam MM, Geula C, Cosgrove R, Mash D, Brimijoin S
Brain research 1991 Jan 25;539(2):233-8
Brain research 1991 Jan 25;539(2):233-8
Monoclonal antibodies to human brain acetylcholinesterase: properties and applications.
Rakonczay Z, Brimijoin S
Cellular and molecular neurobiology 1988 Mar;8(1):85-93
Cellular and molecular neurobiology 1988 Mar;8(1):85-93
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
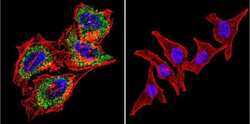
- Experimental details
- Immunofluorescent analysis of Acetylcholinesterase using Anti-Acetylcholinesterase Monoclonal Antibody (HR2) (Product # MA3-042) shows staining in Hela Cells. Acetylcholinesterase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Acetylcholinesterase (Product # MA3-042) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
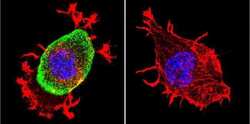
- Experimental details
- Immunofluorescent analysis of Acetylcholinesterase using Anti-Acetylcholinesterase Monoclonal Antibody (HR2) (Product # MA3-042) shows staining in Neuro-2a Cells. Acetylcholinesterase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Acetylcholinesterase (Product # MA3-042) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
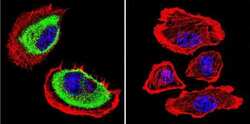
- Experimental details
- Immunofluorescent analysis of Acetylcholinesterase using Anti-Acetylcholinesterase Monoclonal Antibody (HR2) (Product # MA3-042) shows staining in U251 Cells. Acetylcholinesterase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Acetylcholinesterase (Product # MA3-042) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
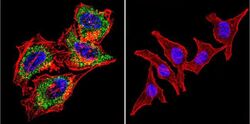
- Experimental details
- Immunofluorescent analysis of Acetylcholinesterase using Anti-Acetylcholinesterase Monoclonal Antibody (HR2) (Product # MA3-042) shows staining in Hela Cells. Acetylcholinesterase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Acetylcholinesterase (Product # MA3-042) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
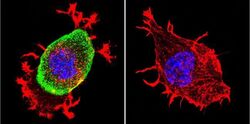
- Experimental details
- Immunofluorescent analysis of Acetylcholinesterase using Anti-Acetylcholinesterase Monoclonal Antibody (HR2) (Product # MA3-042) shows staining in Neuro-2a Cells. Acetylcholinesterase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Acetylcholinesterase (Product # MA3-042) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
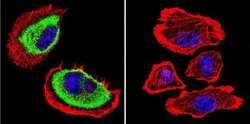
- Experimental details
- Immunofluorescent analysis of Acetylcholinesterase using Anti-Acetylcholinesterase Monoclonal Antibody (HR2) (Product # MA3-042) shows staining in U251 Cells. Acetylcholinesterase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Acetylcholinesterase (Product # MA3-042) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
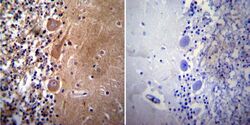
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized human Cerebellum tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a mouse monoclonal antibody recognizing Acetylcholinesterase (Product # MA3-042) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
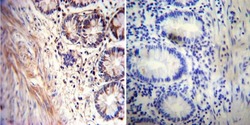
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized human Rectum tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing Acetylcholinesterase (Product # MA3-042) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
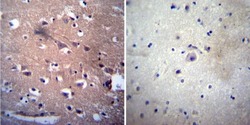
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized human Brain tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Acetylcholinesterase (Product # MA3-042) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
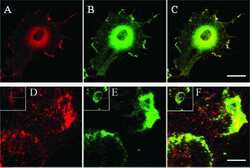
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
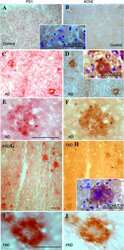
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
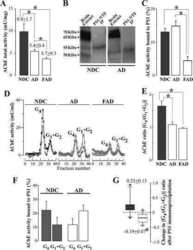
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
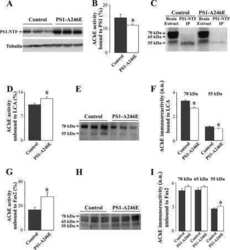
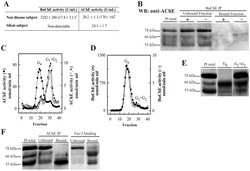
- Figure 1 Plasma AChE levels in healthy controls (wild-type) after BuChE immunodepletion and in BuChE-silent individuals. ( A ) Control plasma was immunoprecipitated with anti-BuChE antibody and cholinesterase activity levels determined before b and after a immunoprecipitation (n = 6; 46+-4 yrs). AChE activity level in plasma from BuChE-silent individuals is also shown (n = 3; 30+-5 yrs). The anti-BuChE antibody does not immunoprecipitate AChE in BuChE-silent plasma (not shown). Values are means +- SEM. ( B ) Immunoprecipitation of control plasma with antibody, followed by immunoblotting with the anti-AChE antibody, N-19. The presence (+) or absence (-) of the anti-BuChE antibody linked to the resin is indicated in the top margin. Prior to electrophoretic analysis, proteins abundant in plasma were depleted by immunoaffinity-based protein subtraction chromatography with IgY microbeads (Seppro(tm)). The anti-BuChE antibody does not immunoprecipitate AChE. Extracts incubated with protein A-Sepharose, in the absence of the antibody, were analyzed in parallel as negative controls. ( C ) Representative profiles of AChE and ( D ) BuChE molecular forms (G 4 = tetramers; G 1 +G 2 = monomers and dimers) in control plasma samples before (*) and after (*) BuChE-immunoprecipitation, and in BuChE-silent plasma (^). ( E ) Representative immunoblot of individual AChE G 4 and G 1 +G 2 peak-fractions separated by sucrose gradient centrifugation from control plasma and detected with the N-19 ant
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
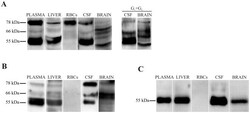
- Figure 3 Immunodetection of AChE subunit variants in human plasma, liver, RBCs, CSF and brain (frontal cortex). Samples were immunoblotted with three anti-AChE antibodies, ( A ) the N-terminal N-19, which recognizes all variants; ( B ) the C-terminal ab31276, which recognizes only AChE-T subunits; and ( C ) the anti- AChE-R antibody directed at the unique C-terminus of AChE-R. Representative immunoblot with the N-19 antibody of individual G 1 +G 2 peak fractions separated by sucrose gradient centrifugation from CSF and brain are included (A, right panel).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
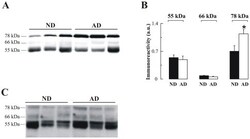
- Figure 5 Altered AChE immunoreactivity in plasma of non-demented controls (ND) and Alzheimer's disease (AD) patients. ( A ) Representative blot of plasma-AChE from AD and ND using the antibody N-19, and ( B ) densitometric quantification of the AChE-immunoreactive bands, expressed in arbitrary units (a u.), from 15 ND (#; n = 15) and 14 AD (#; n = 14) subjects. Proteins abundant in plasma were depleted by immunoaffinity-based protein subtraction chromatography with IgY microbeads (Seppro(tm)) and equivalent amounts of protein were loaded in each lane. Columns represent means +- SEM * p = 0.02 significantly different from NDs as assessed by Student's t test. ( C ) Representative blot of plasma AChE detected with the anti-AChE antibody to the C-terminal, ab31276.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
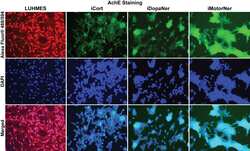
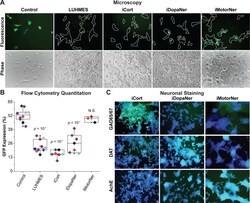
- Fig 3 iPSC-derived neurons repress HIV expression. ( A ) 60,000 hmuglia/HIV HC69 cells were plated in the presence of 0.5 x 10 6 LUHMES-derived neurons (as positive control) or 0.5 x 10 6 iPSC-derived GABAergic cortical (iCort), dopaminergic (iDopaNer) or motor neurons (iMotorNer). HIV expression was evaluated after 24 h by fluorescence microscopy. Microglia identified by phase contrast microscopy are outlined by the white contours. ( B ) Flow cytometric analysis of microglial cell GFP expression. The p -values of pair-sample, Student's t -tests comparing the microglial cells cultured alone or in the presence of neurons are shown. Individual independent experiments are color coded ( n = number of independent samples). N.S.: non- s ignificant. ( C ) Identification of differentiation of iPSC-derived neurons. Super-imposed images of DAPI stained nuclei (blue) and Alexa-Fluor 488 stained neuronal antigens (green) are shown. Neurons were stained with antibodies against GAD65/67, DAT, and AchE and Alexa Fluor 488-conjugated anti-rabbit secondary antibody.
 Explore
Explore Validate
Validate Learn
Learn ELISA
ELISA Immunocytochemistry
Immunocytochemistry