Antibody data
- Antibody Data
- Antigen structure
- References [40]
- Comments [0]
- Validations
- Other assay [22]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-7178-81 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- IL-17A Monoclonal Antibody (eBio64CAP17), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The eBio64CAP17 antibody reacts with human IL-17A; the antibody has been reported to cross react with Rhesus monkey IL-17A, as verified by intracellular staining experiments. The eBio64CAP17 antibody is a neutralizing antibody. This antibody has been shown to have no reactivity to human IL-17F. Reactivity to other members of the IL-17 family has not been evaluated. Interleukin-17A (IL-17A) is a CD4+ T cell-derived cytokine that promotes inflammatory responses in cell lines and is elevated in rheumatoid arthritis, asthma, multiple sclerosis, psoriasis, and transplant rejection. The cDNA encoding human IL-17A was isolated from a library of CD4+ T cells; the encoded protein exhibits 72 percent amino acid identity with HVS13 , an open reading frame from a T lymphotropic Herpesvirus saimiri, and 63 percent with mouse CTLA-8 (cytotoxic T-lymphocyte associated antigen-8). Human IL-17A exists as glycosylated 20-30 kD homodimers. High levels of IL-17A homodimer are produced by activated peripheral blood CD4+ T-cells. IL-17A enhances expression of the intracellular adhesion molecule-1 (ICAM-1) in human fibroblasts. Human IL-17A also stimulates epithelial, endothelial, or fibroblastic cells to secrete IL-6, IL-8, G-CSF, and PGE2. In the presence of human IL-17A, fibroblasts can sustain the proliferation of CD34+ hematopoietic progenitors and induce maturation into neutrophils. Mouse, rat, and human IL-17A can induce IL-6 secretion in mouse stromal cells, indicating that all homologs can recognize the mouse IL-17A receptor. IL-23-dependent, IL-17A-producing CD4+ T cells (Th-17 cells) have been identified as a unique subset of Th cells that develops along a pathway that is distinct from the Th1- and Th2- cell differentiation pathways. The hallmark effector molecules of Th1 and Th2 cells, e.g., IFN-g and IL-4, have each been found to negatively regulate the generation of these Th-17 cells. Additionally, activated human CD4+ T cells have been found to produce the IL-17A/F heterodimer, as well as the corresponding homodimers. In comparing the relative potency of IL-17A, IL-17F, and IL-17A/F, all three were found to induce GRO-a secretion; IL-17A was most potent, followed by IL-17A/F heterodimer, then IL-17F (100fold lower than IL-17A). eBio64CAP17 can be used to detect IL-17 heterodimers by immunoprecipitation followed by immunoblot withH17F10A7 anti-IL17F monoclonal antibody. The eBio64CAP17 has been shown to react to rhesus and marmoset primates. Applications Reported: This eBio64CAP17 antibody has been reported for use in intracellular staining followed by flow cytometric analysis, immunoprecipitation, IHC frozen, ELISA, and ELISPOT. (Fluorochrome conjugated eBio64CAP17 is recommended for use in intracellular flow cytometry). Applications Tested: The Affinity Purified eBio64CAP17 antibody has been tested as the capture antibody in a sandwich ELISA for measurement of human IL-17A protein levels, in combination with the biotinylated eBio64DEC17 antibody (13-7179) for detection and recombinant human IL-17A (14-8179) as the standard. A suitable range of concentrations of this antibody for ELISA capture is 0.5 - 2.0 µg/mL. A standard curve consisting of doubling dilutions of the recombinant standard over the range of 1000 pg/mL - 8 pg/mL should be included in each ELISA plate. The Functional Grade Purified eBio64CAP17 antibody has been tested by LAL assay to verify lowest endotoxin levels and has been tested for neutralization of IL-17A bioactivity and for ELISPOT capture. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- eBio64CAP17
- Vial size
- 50 μg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references FABP4 facilitates inflammasome activation to induce the Treg/Th17 imbalance in preeclampsia via forming a positive feedback with IL-17A.
Th2 Biased Immunity With Altered B Cell Profiles in Circulation of Patients With Sporotrichosis Caused by Sporothrix globosa.
Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens.
Allogeneic ADSCs induce CD8 T cell-mediated cytotoxicity and faster cell death after exposure to xenogeneic serum or proinflammatory cytokines.
The Effects of High Mobility Group Box-1 Protein on Peripheral Treg/Th17 Balance in Patients with Atherosclerosis.
Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection.
P2X7R mutation disrupts the NLRP3-mediated Th program and predicts poor cardiac allograft outcomes.
Application of a whole blood mycobacterial growth inhibition assay to study immunity against Mycobacterium tuberculosis in a high tuberculosis burden population.
Balancing Trained Immunity with Persistent Immune Activation and the Risk of Simian Immunodeficiency Virus Infection in Infant Macaques Vaccinated with Attenuated Mycobacterium tuberculosis or Mycobacterium bovis BCG Vaccine.
Laparoscopic Technique for Serial Collection of Para-Colonic, Left Colic, and Inferior Mesenteric Lymph Nodes in Macaques.
A molecular signature of preclinical rheumatoid arthritis triggered by dysregulated PTPN22.
Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques.
Downregulation of RUNX3 moderates the frequency of Th17 and Th22 cells in patients with psoriasis.
Altered expression of miR-92a correlates with Th17 cell frequency in patients with primary biliary cirrhosis.
Transcriptional Profiling of Th2 Cells Identifies Pathogenic Features Associated with Asthma.
Definition of a pool of epitopes that recapitulates the T cell reactivity against major house dust mite allergens.
COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis.
Fiber composite slices for multiplexed immunoassays.
Differential requirement for P2X7R function in IL-17 dependent vs. IL-17 independent cellular immune responses.
CCL20 Secretion from the Nucleus Pulposus Improves the Recruitment of CCR6-Expressing Th17 Cells to Degenerated IVD Tissues.
Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques.
Human regulatory T cells do not suppress the antitumor immunity in the bone marrow: a role for bone marrow stromal cells in neutralizing regulatory T cells.
Protein microarray analysis reveals BAFF-binding autoantibodies in systemic lupus erythematosus.
Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion.
Gluten-sensitive enteropathy coincides with decreased capability of intestinal T cells to secrete IL-17 and IL-22 in a macaque model for celiac disease.
IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques.
Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection.
Prolonged tenofovir treatment of macaques infected with K65R reverse transcriptase mutants of SIV results in the development of antiviral immune responses that control virus replication after drug withdrawal.
Licensing virus-specific T cells to secrete the neutrophil attracting chemokine CXCL-8 during hepatitis B virus infection.
Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis.
Virus infection stages and distinct Th1 or Th17/Th22 T-cell responses in malaria/SHIV coinfection correlate with different outcomes of disease.
Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients.
IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells.
CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation.
A comprehensive ex vivo functional analysis of human NKT cells reveals production of MIP1-α and MIP1-β, a lack of IL-17, and a Th1-bias in males.
Molecular determinants of T cell epitope recognition to the common Timothy grass allergen.
CD49d provides access to "untouched" human Foxp3+ Treg free of contaminating effector cells.
A monoclonal antibody selection for immunohistochemical examination of lymphoid tissues from non-human primates.
Distinct regulation of interleukin-17 in human T helper lymphocytes.
Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation.
Chang GP, Yang XL, Liu W, Lin S, Yang SL, Zhao MY
Molecular therapy. Nucleic acids 2021 Jun 4;24:743-754
Molecular therapy. Nucleic acids 2021 Jun 4;24:743-754
Th2 Biased Immunity With Altered B Cell Profiles in Circulation of Patients With Sporotrichosis Caused by Sporothrix globosa.
Zu J, Yao L, Song Y, Cui Y, Guan M, Chen R, Zhen Y, Li S
Frontiers in immunology 2020;11:570888
Frontiers in immunology 2020;11:570888
Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens.
Chang SH, Kim HJ, Park CG
Cells 2020 May 18;9(5)
Cells 2020 May 18;9(5)
Allogeneic ADSCs induce CD8 T cell-mediated cytotoxicity and faster cell death after exposure to xenogeneic serum or proinflammatory cytokines.
Chang SH, Park CG
Experimental & molecular medicine 2019 Mar 11;51(3):1-10
Experimental & molecular medicine 2019 Mar 11;51(3):1-10
The Effects of High Mobility Group Box-1 Protein on Peripheral Treg/Th17 Balance in Patients with Atherosclerosis.
Ding JW, Zhou T, Zheng XX, Wang XA, Tong XH, Luo CY, Zhang ZQ, Yu B
Acta Cardiologica Sinica 2018 Sep;34(5):399-408
Acta Cardiologica Sinica 2018 Sep;34(5):399-408
Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection.
Dillen CA, Pinsker BL, Marusina AI, Merleev AA, Farber ON, Liu H, Archer NK, Lee DB, Wang Y, Ortines RV, Lee SK, Marchitto MC, Cai SS, Ashbaugh AG, May LS, Holland SM, Freeman AF, Miller LG, Yeaman MR, Simon SI, Milner JD, Maverakis E, Miller LS
The Journal of clinical investigation 2018 Mar 1;128(3):1026-1042
The Journal of clinical investigation 2018 Mar 1;128(3):1026-1042
P2X7R mutation disrupts the NLRP3-mediated Th program and predicts poor cardiac allograft outcomes.
D'Addio F, Vergani A, Potena L, Maestroni A, Usuelli V, Ben Nasr M, Bassi R, Tezza S, Dellepiane S, El Essawy B, Iascone M, Iacovoni A, Borgese L, Liu K, Visner G, Dhe-Paganon S, Corradi D, Abdi R, Starling RC, Folli F, Zuccotti GV, Sayegh MH, Heeger PS, Chandraker A, Grigioni F, Fiorina P
The Journal of clinical investigation 2018 Aug 1;128(8):3490-3503
The Journal of clinical investigation 2018 Aug 1;128(8):3490-3503
Application of a whole blood mycobacterial growth inhibition assay to study immunity against Mycobacterium tuberculosis in a high tuberculosis burden population.
Baguma R, Penn-Nicholson A, Smit E, Erasmus M, Day J, Makhethe L, de Kock M, Hughes EJ, van Rooyen M, Pienaar B, Stone L, Hanekom W, Brennan MJ, Wallis RS, Hatherill M, Scriba TJ
PloS one 2017;12(9):e0184563
PloS one 2017;12(9):e0184563
Balancing Trained Immunity with Persistent Immune Activation and the Risk of Simian Immunodeficiency Virus Infection in Infant Macaques Vaccinated with Attenuated Mycobacterium tuberculosis or Mycobacterium bovis BCG Vaccine.
Jensen K, Dela Pena-Ponce MG, Piatak M Jr, Shoemaker R, Oswald K, Jacobs WR Jr, Fennelly G, Lucero C, Mollan KR, Hudgens MG, Amedee A, Kozlowski PA, Estes JD, Lifson JD, Van Rompay KK, Larsen M, De Paris K
Clinical and vaccine immunology : CVI 2017 Jan;24(1)
Clinical and vaccine immunology : CVI 2017 Jan;24(1)
Laparoscopic Technique for Serial Collection of Para-Colonic, Left Colic, and Inferior Mesenteric Lymph Nodes in Macaques.
Smedley J, Macalister R, Wangari S, Gathuka M, Ahrens J, Iwayama N, May D, Bratt D, O'Connor M, Munson P, Koday M, Lifson J, Fuller DH
PloS one 2016;11(6):e0157535
PloS one 2016;11(6):e0157535
A molecular signature of preclinical rheumatoid arthritis triggered by dysregulated PTPN22.
Chang HH, Liu GY, Dwivedi N, Sun B, Okamoto Y, Kinslow JD, Deane KD, Demoruelle MK, Norris JM, Thompson PR, Sparks JA, Rao DA, Karlson EW, Hung HC, Holers VM, Ho IC
JCI insight 2016 Oct 20;1(17):e90045
JCI insight 2016 Oct 20;1(17):e90045
Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques.
Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR, Ruden R, Cirrincione LR, Chan J, Lin PL, Flynn JL
Infection and immunity 2016 May;84(5):1301-1311
Infection and immunity 2016 May;84(5):1301-1311
Downregulation of RUNX3 moderates the frequency of Th17 and Th22 cells in patients with psoriasis.
Fu D, Song X, Hu H, Sun M, Li Z, Tian Z
Molecular medicine reports 2016 Jun;13(6):4606-12
Molecular medicine reports 2016 Jun;13(6):4606-12
Altered expression of miR-92a correlates with Th17 cell frequency in patients with primary biliary cirrhosis.
Liang DY, Hou YQ, Luo LJ, Ao L
International journal of molecular medicine 2016 Jul;38(1):131-8
International journal of molecular medicine 2016 Jul;38(1):131-8
Transcriptional Profiling of Th2 Cells Identifies Pathogenic Features Associated with Asthma.
Seumois G, Zapardiel-Gonzalo J, White B, Singh D, Schulten V, Dillon M, Hinz D, Broide DH, Sette A, Peters B, Vijayanand P
Journal of immunology (Baltimore, Md. : 1950) 2016 Jul 15;197(2):655-64
Journal of immunology (Baltimore, Md. : 1950) 2016 Jul 15;197(2):655-64
Definition of a pool of epitopes that recapitulates the T cell reactivity against major house dust mite allergens.
Hinz D, Oseroff C, Pham J, Sidney J, Peters B, Sette A
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2015 Oct;45(10):1601-12
Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2015 Oct;45(10):1601-12
COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis.
Watkin LB, Jessen B, Wiszniewski W, Vece TJ, Jan M, Sha Y, Thamsen M, Santos-Cortez RL, Lee K, Gambin T, Forbes LR, Law CS, Stray-Pedersen A, Cheng MH, Mace EM, Anderson MS, Liu D, Tang LF, Nicholas SK, Nahmod K, Makedonas G, Canter DL, Kwok PY, Hicks J, Jones KD, Penney S, Jhangiani SN, Rosenblum MD, Dell SD, Waterfield MR, Papa FR, Muzny DM, Zaitlen N, Leal SM, Gonzaga-Jauregui C, Baylor-Hopkins Center for Mendelian Genomics, Boerwinkle E, Eissa NT, Gibbs RA, Lupski JR, Orange JS, Shum AK
Nature genetics 2015 Jun;47(6):654-60
Nature genetics 2015 Jun;47(6):654-60
Fiber composite slices for multiplexed immunoassays.
Kim J, Bae S, Song S, Chung K, Kwon S
Biomicrofluidics 2015 Jul;9(4):044109
Biomicrofluidics 2015 Jul;9(4):044109
Differential requirement for P2X7R function in IL-17 dependent vs. IL-17 independent cellular immune responses.
Sullivan JA, Jankowska-Gan E, Shi L, Roenneburg D, Hegde S, Greenspan DS, Wilkes DS, Denlinger LC, Burlingham WJ
American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014 Jul;14(7):1512-22
American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2014 Jul;14(7):1512-22
CCL20 Secretion from the Nucleus Pulposus Improves the Recruitment of CCR6-Expressing Th17 Cells to Degenerated IVD Tissues.
Zhang W, Nie L, Wang Y, Wang XP, Zhao H, Dongol S, Maharjan S, Cheng L
PloS one 2013;8(6):e66286
PloS one 2013;8(6):e66286
Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques.
Canary LA, Vinton CL, Morcock DR, Pierce JB, Estes JD, Brenchley JM, Klatt NR
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 15;190(6):2959-65
Journal of immunology (Baltimore, Md. : 1950) 2013 Mar 15;190(6):2959-65
Human regulatory T cells do not suppress the antitumor immunity in the bone marrow: a role for bone marrow stromal cells in neutralizing regulatory T cells.
Guichelaar T, Emmelot ME, Rozemuller H, Martini B, Groen RW, Storm G, Lokhorst HM, Martens AC, Mutis T
Clinical cancer research : an official journal of the American Association for Cancer Research 2013 Mar 15;19(6):1467-75
Clinical cancer research : an official journal of the American Association for Cancer Research 2013 Mar 15;19(6):1467-75
Protein microarray analysis reveals BAFF-binding autoantibodies in systemic lupus erythematosus.
Price JV, Haddon DJ, Kemmer D, Delepine G, Mandelbaum G, Jarrell JA, Gupta R, Balboni I, Chakravarty EF, Sokolove J, Shum AK, Anderson MS, Cheng MH, Robinson WH, Browne SK, Holland SM, Baechler EC, Utz PJ
The Journal of clinical investigation 2013 Dec;123(12):5135-45
The Journal of clinical investigation 2013 Dec;123(12):5135-45
Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion.
Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM
The Journal of allergy and clinical immunology 2013 Aug;132(2):297-304.e3
The Journal of allergy and clinical immunology 2013 Aug;132(2):297-304.e3
Gluten-sensitive enteropathy coincides with decreased capability of intestinal T cells to secrete IL-17 and IL-22 in a macaque model for celiac disease.
Xu H, Feely SL, Wang X, Liu DX, Borda JT, Dufour J, Li W, Aye PP, Doxiadis GG, Khosla C, Veazey RS, Sestak K
Clinical immunology (Orlando, Fla.) 2013 Apr;147(1):40-49
Clinical immunology (Orlando, Fla.) 2013 Apr;147(1):40-49
IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques.
Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS
Mucosal immunology 2012 Nov;5(6):658-69
Mucosal immunology 2012 Nov;5(6):658-69
Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection.
Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM
Mucosal immunology 2012 Nov;5(6):646-57
Mucosal immunology 2012 Nov;5(6):646-57
Prolonged tenofovir treatment of macaques infected with K65R reverse transcriptase mutants of SIV results in the development of antiviral immune responses that control virus replication after drug withdrawal.
Van Rompay KK, Trott KA, Jayashankar K, Geng Y, LaBranche CC, Johnson JA, Landucci G, Lipscomb J, Tarara RP, Canfield DR, Heneine W, Forthal DN, Montefiori D, Abel K
Retrovirology 2012 Jul 17;9:57
Retrovirology 2012 Jul 17;9:57
Licensing virus-specific T cells to secrete the neutrophil attracting chemokine CXCL-8 during hepatitis B virus infection.
Gehring AJ, Koh S, Chia A, Paramasivam K, Chew VS, Ho ZZ, Lee KH, Maini MK, Madhavan K, Lim SG, Bertoletti A
PloS one 2011;6(8):e23330
PloS one 2011;6(8):e23330
Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis.
Morbach H, Wiegering V, Richl P, Schwarz T, Suffa N, Eichhorn EM, Eyrich M, Girschick HJ
Arthritis and rheumatism 2011 Nov;63(11):3458-66
Arthritis and rheumatism 2011 Nov;63(11):3458-66
Virus infection stages and distinct Th1 or Th17/Th22 T-cell responses in malaria/SHIV coinfection correlate with different outcomes of disease.
Ryan-Payseur B, Ali Z, Huang D, Chen CY, Yan L, Wang RC, Collins WE, Wang Y, Chen ZW
The Journal of infectious diseases 2011 Nov;204(9):1450-62
The Journal of infectious diseases 2011 Nov;204(9):1450-62
Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients.
Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS
Journal of immunology (Baltimore, Md. : 1950) 2011 Jul 15;187(2):1023-30
Journal of immunology (Baltimore, Md. : 1950) 2011 Jul 15;187(2):1023-30
IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells.
Hebel K, Rudolph M, Kosak B, Chang HD, Butzmann J, Brunner-Weinzierl MC
Journal of immunology (Baltimore, Md. : 1950) 2011 Dec 1;187(11):5627-35
Journal of immunology (Baltimore, Md. : 1950) 2011 Dec 1;187(11):5627-35
CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation.
de Wit J, Souwer Y, van Beelen AJ, de Groot R, Muller FJ, Klaasse Bos H, Jorritsma T, Kapsenberg ML, de Jong EC, van Ham SM
Blood 2011 Dec 1;118(23):6107-14
Blood 2011 Dec 1;118(23):6107-14
A comprehensive ex vivo functional analysis of human NKT cells reveals production of MIP1-α and MIP1-β, a lack of IL-17, and a Th1-bias in males.
Snyder-Cappione JE, Tincati C, Eccles-James IG, Cappione AJ, Ndhlovu LC, Koth LL, Nixon DF
PloS one 2010 Nov 3;5(11):e15412
PloS one 2010 Nov 3;5(11):e15412
Molecular determinants of T cell epitope recognition to the common Timothy grass allergen.
Oseroff C, Sidney J, Kotturi MF, Kolla R, Alam R, Broide DH, Wasserman SI, Weiskopf D, McKinney DM, Chung JL, Petersen A, Grey H, Peters B, Sette A
Journal of immunology (Baltimore, Md. : 1950) 2010 Jul 15;185(2):943-55
Journal of immunology (Baltimore, Md. : 1950) 2010 Jul 15;185(2):943-55
CD49d provides access to "untouched" human Foxp3+ Treg free of contaminating effector cells.
Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rötzschke O, Falk K
Blood 2009 Jan 22;113(4):827-36
Blood 2009 Jan 22;113(4):827-36
A monoclonal antibody selection for immunohistochemical examination of lymphoid tissues from non-human primates.
Kap YS, van Meurs M, van Driel N, Koopman G, Melief MJ, Brok HP, Laman JD, 't Hart BA
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2009 Dec;57(12):1159-67
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2009 Dec;57(12):1159-67
Distinct regulation of interleukin-17 in human T helper lymphocytes.
Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ
Arthritis and rheumatism 2007 Sep;56(9):2936-46
Arthritis and rheumatism 2007 Sep;56(9):2936-46
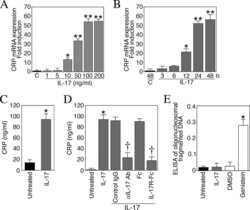
Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation.
Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B
The Journal of biological chemistry 2007 Sep 14;282(37):27229-27238
The Journal of biological chemistry 2007 Sep 14;282(37):27229-27238
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
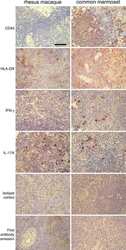
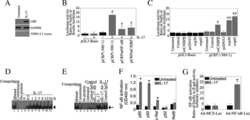
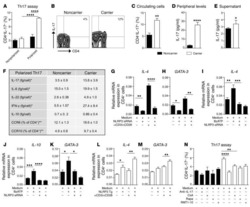
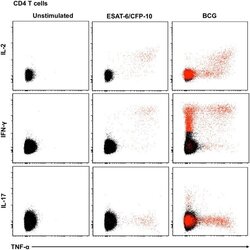
- Fig 5 Representative flow cytometry plots of cytokine expression by CD4 T cells. CD4 T cells expressing IFN-gamma, IL-2, TNF-alpha and/or IL-17 upon stimulation with BCG or ESAT-6/CFP-10 peptide pools for 12 hours, compared to an unstimulated control sample. The plots represent cytokine-positive cells (red) overlayed onto the background of the entire CD4 T cell parent population (black). Similar assessment was also performed for CD8 and gammadelta T cells (data not shown).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
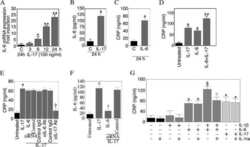
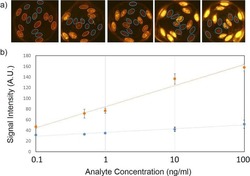
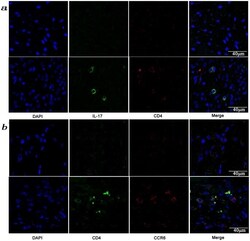
- Figure 3 Presence of IL-17-producing cells in the degenerated IVD tissues. Fifty IVD tissue samples (Group P, n = 20 and Group E, n = 30) from disc degeneration patients and 3 from scoliosis patients were analyzed. In Group P or the control group, there were few or no positive cells (data not shown). The representative results of group E are shown. (a), IL-17-producing cells were detected by a rabbit anti-IL-17 polyclonal antibody and a mouse anti-CD4 monoclonal Ab, followed by secondary staining with an Alexa 488-conjugated donkey anti-rabbit and an Alexa 568-conjugated donkey anti-mouse IgG. DAPI mounting medium was used for nuclear staining. (b), surface CCR6 expression on the cells was detected with a rabbit anti CD4 monoclonal Ab and a mouse anti-CCR6 monoclonal Ab, followed by secondary staining with an Alexa 488-conjugated donkey anti-rabbit and an Alexa 568-conjugated donkey anti-mouse IgG. DAPI mounting medium was used for nuclear staining. In the top panel, green and red represents the expression of IL-17 and CD4, respectively, and the double-stained cells represent the IL-17-producing cells. In the bottom panel, green and red represents the expression of CD4 and CCR6, respectively, and the double-stained cells demonstrate the surface expression of CCR6 on T lymphocytes.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
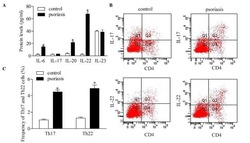
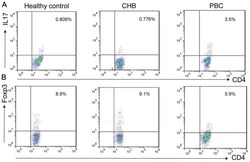
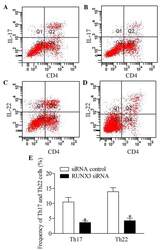
- Figure 5 Inhibition of RUNX3 regulates the frequencies of Th17 and Th22 in CD4 + T cells from patients with psoriasis. The frequencies of Th17 and Th22 cells in CD4 + T cells from patients with psoriasis transfected with RUNX3 siRNA or an siRNA control was detected using flow cytometry. (A and B) Flow cytometric analysis of Th17 in CD4 + T cells from patients with psoriasis. The percentages of cells in the Q2 region represent the percentage of Th17 cells. (C and D) Flow cytometric analysis of Th22 in CD4 + T cells from patients with psoriasis. The percentages of cells in the Q2 region represent the percentage of Th22 cells. (E) Percentages of Th17 and Th22 cells in CD4 + T cells from patients with psoriasis transfected with RUNX3 siRNA or an siRNA control. Data are presented as the mean +- standard deviation of three experiments. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
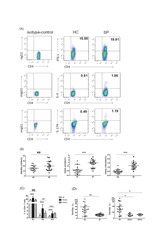
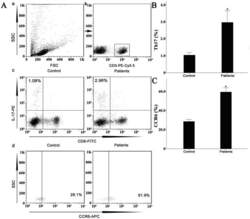
- Figure 7 Circulating percentages of Th17 cells and CCR6-positive cells in peripheral blood are increased in IVD degenerated patients when compared with controls. Heparinized peripheral whole blood cells from 20 patients and 15 healthy controls were stimulated with phorbol myristate acetate (PMA), ionomycin, and monensin for 4 h and subsequently stained with fluorochrome-labeled antibodies as described in Materials and Methods. A(a) Lymphocytes were gated by flow cytometry. A(b) CD3 + T subsets were gated by flow cytometry; the plots in the inset box represent the CD3 + T cells. A(c) Representative IL-17 expression levels in the CD3 + CD8 - T subsets (CD4 + T subsets) from each group are shown. The percentages of positive cells are shown in the upper left panels. A(d) Representative surface CCR6 expression levels on the CD3 + CD8 - IL-17 + subsets from each group are shown. The percentages of positive cells are shown in the right panel. (B) The percentage of circulating Th17 cells was significantly higher in IVD degenerated patients (2.973+-0.689%) than in the control group (1.039+-0.156%; *, p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
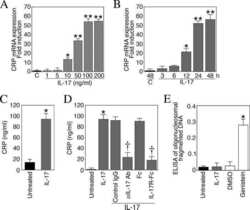
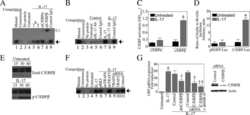
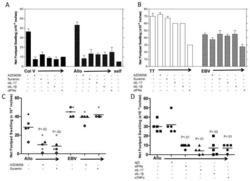
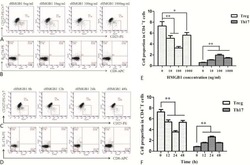
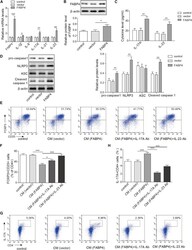
- Figure 4 FABP4 promotes Th17 differentiation of naive T cells via increasing IL-17A/IL-23 release (A) The mRNA levels of cytokines were determined by qRT-PCR. (B) The protein level of FABP4 was detected by western blot. (C) The IL-17A and IL-23 levels in cell culture supernatant were assessed by ELISA. (D) The protein levels of NLRP3 inflammasome components were determined by western blot. Naive T cells were cultured under Treg or Th17 cell-polarizing conditions with or without primary macrophages-CM and neutralizing antibody. (E-H) The percentage of Tregs (E and F) or Th17 cells (G and H) was assessed by FACS. GAPDH and beta-actin were used for normalization in qRT-PCR and western blot, respectively. Data are expressed as representative images or the mean +- SD of n = 3 experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
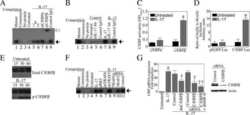
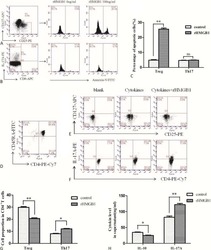
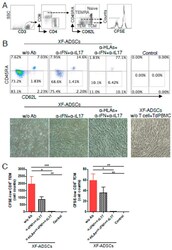
- Figure 6 HLA-blocking antibodies inhibit the production of alloreactive memory-CD8 T cells during allogeneic-antigen stimulation. XF-ADSCs were cultured with CD3 T cells and Td-PBMCs via the direct pathway, after 3 weeks whole cells were harvested and memory-CD8 T cells were analyzed by flow cytometry. ( A ) Scheme for the flow cytometry analysis. ( B ) CD3 T cells were cultured for 3 weeks with or without neutralizing or blocking antibodies during allogeneic-antigen stimulation. A combination of anti-human IL-17A and anti-human IFN-gamma antibodies was used to neutralize pro-inflammatory cytokines. A combination of anti-HLA-ABC, anti-HLA-DR and anti-HLA-DQ antibodies was used to block HLAs. The dot plots showed the population of memory-CD8 T cells on the 21st day after allogeneic-antigen stimulation, compared with the control. ( C ) Graphical representation of the number of CFSE-low memory-CD8 T cells corresponding to (B) ( n = 7 (each CD8 TEM), n = 6 (each CD8 TCM)). The control does not contain XF-ADSCs and consist only of CD3 T cells and Td-PBMCs from healthy donor. The data were repeated at least three times; *, p < 0.05; **, p < 0.01; ***, p < 0.001. alpha-IFN+alpha-IL17: combination of anti-human IFN-gamma and anti-human IL-17A antibodies; alpha-HLAs: combination of anti-HLA-ABC, HLA-DR and anti-HLA-DQ antibodies.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Fig. 5 Human leukocyte antigens (HLAs) on allogeneic adipose-derived mesenchymal stem cells grown in xeno-free medium (XF-ADSCs) are the major cause of T cell-mediated cytotoxicity. a XF-ADSCs were subjected to allogeneic antigen stimulation for 21-24 days with antibodies against interferon-gamma (IFN-gamma), interleukin (IL)-17A, HLA-ABC, HLA-DR, and HLA-DQ (all at 1 mug/ml) in xeno-free medium containing 5% autologous serum. The effects of the XF-ADSCs with antibodies on T cell-mediated cytotoxicity were evaluated in comparison with those of the XF-ADSCs not containing antibodies. For cell death analysis, the collected XF-ADSCs were stained with anti-CD73 and anti-CD90 antibodies, annexin V, and propidium iodide (PI), and the cell death rate was analyzed by flow cytometry. b Graphical representation of XF-ADSC death corresponding to ( a ) ( n = 21 (untreated), n = 7 (for each sample)); *** p < 0.001. Td-PBMCs T cell-depleted T cell-depleted peripheral blood mononuclear cells
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Other assay
Other assay