MA1-025
antibody from Invitrogen Antibodies
Targeting: NFATC2
NF-ATP, NFAT1, NFATp
 Western blot
Western blot Immunocytochemistry
Immunocytochemistry Immunoprecipitation
Immunoprecipitation Immunohistochemistry
Immunohistochemistry Gel shift
Gel shift Chromatin Immunoprecipitation
Chromatin Immunoprecipitation Other assay
Other assayAntibody data
- Antibody Data
- Antigen structure
- References [36]
- Comments [0]
- Validations
- Immunocytochemistry [10]
- Immunohistochemistry [3]
- Chromatin Immunoprecipitation [2]
- Other assay [14]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-025 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- NFATC2 Monoclonal Antibody (25A10.D6.D2)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- MA1-025 detects NFATc2 (NFAT1) from mouse, rat and human tissues. This antibody does not cross react with NFAT2 (NFATc, NFATc1). MA1-025 has been successfully used in Western blot, ChIP, immunocytochemistry, immunoprecipitation, immunofluorescence, immunohistochemistry (frozen & paraffin), and gel shift procedures. By Western blot, this antibody detects an ~140 kDa protein representing phosphorylated NFAT1 in resting immune cells, and an ~120 kDa protein in stimulated cells that represents fully-dephosphorylated NFAT1. The MA1-025 immunogen is a synthetic peptide corresponding to residues A(51) I S S P S G L A Y P D D V L D Y G L(69) of mouse NFAT1-A, B and C isoforms. The sequence of this N-terminal peptide is ~70% homologous with human NFAT1. Antibodies to this protein were previously sold as part of a Thermo Scientific Cellomics High Content Screening Kit. This replacement antibody is now recommended for researchers who need an antibody for high content cell-based assays. It has been thoroughly tested and validated for cellular immunofluorescence (IF) applications. Further optimization, including the selection of the most appropriate fluorescent DyLight conjugated secondary antibody, may have to be performed for your high content assay. MA1-025 can be used with blocking peptide PEP-062.
- Reactivity
- Human, Mouse, Rat
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 25A10.D6.D2
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references X-ray irradiation triggers immune response in human T-lymphocytes via store-operated Ca2+ entry and NFAT activation.
Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation.
SIRT2 deacetylase regulates the activity of GSK3 isoforms independent of inhibitory phosphorylation.
Unique properties of TCR-activated p38 are necessary for NFAT-dependent T-cell activation.
The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression.
Dual-specificity tyrosine-phosphorylation regulated kinase 1A Gene Transcription is regulated by Myocyte Enhancer Factor 2D.
Deficiency of N-myristoylation reveals calcineurin activity as regulator of IFN-γ-producing γδ T cells.
Autocrine lysophosphatidic acid signaling activates β-catenin and promotes lung allograft fibrosis.
Toll-Like Receptors Promote Mitochondrial Translocation of Nuclear Transcription Factor Nuclear Factor of Activated T-Cells in Prolonged Microglial Activation.
Nuclear factor of activated T-cells (NFAT)C2 inhibits Notch receptor signaling in osteoblasts.
Lipocalin 2, the TNF-like receptor TWEAKR and its ligand TWEAK act downstream of NFAT1 to regulate breast cancer cell invasion.
Constitutive nuclear localization of NFAT in Foxp3+ regulatory T cells independent of calcineurin activity.
Cutting edge: Regulatory T cells selectively attenuate, not terminate, T cell signaling by disrupting NF-κB nuclear accumulation in CD4 T cells.
Calcineurin/NFAT signalling inhibits myeloid haematopoiesis.
NFATc1 regulation of TRAIL expression in human intestinal cells.
Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex.
Pivotal Advance: Nonfunctional lung effectors exhibit decreased calcium mobilization associated with reduced expression of ORAI1.
FOXP3 inhibits activation-induced NFAT2 expression in T cells thereby limiting effector cytokine expression.
Proteolytic regulation of nuclear factor of activated T (NFAT) c2 cells and NFAT activity by caspase-3.
Hypertrophy and transcriptional regulation induced in myogenic cell line L6-C5 by an increase of extracellular calcium.
Calcium-dependent activation of interleukin-21 gene expression in T cells.
Nicotine activates nuclear factor of activated T cells c2 (NFATc2) and prevents cell cycle entry in T cells.
Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis.
Interleukin (IL)-15 and IL-2 reciprocally regulate expression of the chemokine receptor CX3CR1 through selective NFAT1- and NFAT2-dependent mechanisms.
TRPC3 channels confer cellular memory of recent neuromuscular activity.
Regulation of the murine Nfatc1 gene by NFATc2.
A T cell-specific enhancer of the human CD40 ligand gene.
The basis for TCR-mediated regulation of the IL-2 receptor alpha chain gene: role of widely separated regulatory elements.
Double-stranded RNA regulates IL-4 expression.
Double-stranded RNA regulates IL-4 expression.
Identification and characterization of a novel nuclear factor of activated T-cells-1 isoform expressed in mouse brain.
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Expression of the transcription factor NFATp in a neuronal cell line and in the murine nervous system.
Tandl D, Sponagel T, Alansary D, Fuck S, Smit T, Hehlgans S, Jakob B, Fournier C, Niemeyer BA, Rödel F, Roth B, Moroni A, Thiel G
The Journal of general physiology 2022 May 2;154(5)
The Journal of general physiology 2022 May 2;154(5)
Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation.
Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, Zhang Y, Aleman-Muench GR, Lewis G, Chen W, Kang S, Luevanos M, Trudler D, Lipton SA, Soroosh P, Teijaro J, de la Torre JC, Arditi M, Karin M, Sanchez-Lopez E
Immunity 2021 Jul 13;54(7):1463-1477.e11
Immunity 2021 Jul 13;54(7):1463-1477.e11
SIRT2 deacetylase regulates the activity of GSK3 isoforms independent of inhibitory phosphorylation.
Sarikhani M, Mishra S, Maity S, Kotyada C, Wolfgeher D, Gupta MP, Singh M, Sundaresan NR
eLife 2018 Mar 5;7
eLife 2018 Mar 5;7
Unique properties of TCR-activated p38 are necessary for NFAT-dependent T-cell activation.
Alam MS, Gaida MM, Debnath S, Tagad HD, Miller Jenkins LM, Appella E, Rahman MJ, Ashwell JD
PLoS biology 2018 Jan;16(1):e2004111
PLoS biology 2018 Jan;16(1):e2004111
The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression.
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li JY, Owens JA, Hsu E, Adams J, Weitzmann MN, Jones RM, Pacifici R
Immunity 2018 Dec 18;49(6):1116-1131.e7
Immunity 2018 Dec 18;49(6):1116-1131.e7
Dual-specificity tyrosine-phosphorylation regulated kinase 1A Gene Transcription is regulated by Myocyte Enhancer Factor 2D.
Wang P, Wang L, Chen L, Sun X
Scientific reports 2017 Aug 3;7(1):7240
Scientific reports 2017 Aug 3;7(1):7240
Deficiency of N-myristoylation reveals calcineurin activity as regulator of IFN-γ-producing γδ T cells.
Rampoldi F, Brunk F, Bonrouhi M, Federico G, Krunic D, Porubsky S, Gröne HJ, Popovic ZV
Journal of leukocyte biology 2017 Apr;101(4):1005-1014
Journal of leukocyte biology 2017 Apr;101(4):1005-1014
Autocrine lysophosphatidic acid signaling activates β-catenin and promotes lung allograft fibrosis.
Cao P, Aoki Y, Badri L, Walker NM, Manning CM, Lagstein A, Fearon ER, Lama VN
The Journal of clinical investigation 2017 Apr 3;127(4):1517-1530
The Journal of clinical investigation 2017 Apr 3;127(4):1517-1530
Toll-Like Receptors Promote Mitochondrial Translocation of Nuclear Transcription Factor Nuclear Factor of Activated T-Cells in Prolonged Microglial Activation.
Ma B, Yu J, Xie C, Sun L, Lin S, Ding J, Luo J, Cai H
The Journal of neuroscience : the official journal of the Society for Neuroscience 2015 Jul 29;35(30):10799-814
The Journal of neuroscience : the official journal of the Society for Neuroscience 2015 Jul 29;35(30):10799-814
Nuclear factor of activated T-cells (NFAT)C2 inhibits Notch receptor signaling in osteoblasts.
Zanotti S, Smerdel-Ramoya A, Canalis E
The Journal of biological chemistry 2013 Jan 4;288(1):624-32
The Journal of biological chemistry 2013 Jan 4;288(1):624-32
Lipocalin 2, the TNF-like receptor TWEAKR and its ligand TWEAK act downstream of NFAT1 to regulate breast cancer cell invasion.
Gaudineau B, Fougère M, Guaddachi F, Lemoine F, de la Grange P, Jauliac S
Journal of cell science 2012 Oct 1;125(Pt 19):4475-86
Journal of cell science 2012 Oct 1;125(Pt 19):4475-86
Constitutive nuclear localization of NFAT in Foxp3+ regulatory T cells independent of calcineurin activity.
Li Q, Shakya A, Guo X, Zhang H, Tantin D, Jensen PE, Chen X
Journal of immunology (Baltimore, Md. : 1950) 2012 May 1;188(9):4268-77
Journal of immunology (Baltimore, Md. : 1950) 2012 May 1;188(9):4268-77
Cutting edge: Regulatory T cells selectively attenuate, not terminate, T cell signaling by disrupting NF-κB nuclear accumulation in CD4 T cells.
Huang YH, Sojka DK, Fowell DJ
Journal of immunology (Baltimore, Md. : 1950) 2012 Feb 1;188(3):947-51
Journal of immunology (Baltimore, Md. : 1950) 2012 Feb 1;188(3):947-51
Calcineurin/NFAT signalling inhibits myeloid haematopoiesis.
Fric J, Lim CX, Koh EG, Hofmann B, Chen J, Tay HS, Mohammad Isa SA, Mortellaro A, Ruedl C, Ricciardi-Castagnoli P
EMBO molecular medicine 2012 Apr;4(4):269-82
EMBO molecular medicine 2012 Apr;4(4):269-82
NFATc1 regulation of TRAIL expression in human intestinal cells.
Wang Q, Zhou Y, Weiss HL, Chow CW, Evers BM
PloS one 2011;6(5):e19882
PloS one 2011;6(5):e19882
Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex.
Ohoka Y, Yokota A, Takeuchi H, Maeda N, Iwata M
Journal of immunology (Baltimore, Md. : 1950) 2011 Jan 15;186(2):733-44
Journal of immunology (Baltimore, Md. : 1950) 2011 Jan 15;186(2):733-44
Pivotal Advance: Nonfunctional lung effectors exhibit decreased calcium mobilization associated with reduced expression of ORAI1.
Arimilli S, Sharma SK, Yammani R, Reid SD, Parks GD, Alexander-Miller MA
Journal of leukocyte biology 2010 Jun;87(6):977-88
Journal of leukocyte biology 2010 Jun;87(6):977-88
FOXP3 inhibits activation-induced NFAT2 expression in T cells thereby limiting effector cytokine expression.
Torgerson TR, Genin A, Chen C, Zhang M, Zhou B, Añover-Sombke S, Frank MB, Dozmorov I, Ocheltree E, Kulmala P, Centola M, Ochs HD, Wells AD, Cron RQ
Journal of immunology (Baltimore, Md. : 1950) 2009 Jul 15;183(2):907-15
Journal of immunology (Baltimore, Md. : 1950) 2009 Jul 15;183(2):907-15
Proteolytic regulation of nuclear factor of activated T (NFAT) c2 cells and NFAT activity by caspase-3.
Wu W, Misra RS, Russell JQ, Flavell RA, Rincón M, Budd RC
The Journal of biological chemistry 2006 Apr 21;281(16):10682-90
The Journal of biological chemistry 2006 Apr 21;281(16):10682-90
Hypertrophy and transcriptional regulation induced in myogenic cell line L6-C5 by an increase of extracellular calcium.
De Arcangelis V, Coletti D, Canato M, Molinaro M, Adamo S, Reggiani C, Naro F
Journal of cellular physiology 2005 Mar;202(3):787-95
Journal of cellular physiology 2005 Mar;202(3):787-95
Calcium-dependent activation of interleukin-21 gene expression in T cells.
Kim HP, Korn LL, Gamero AM, Leonard WJ
The Journal of biological chemistry 2005 Jul 1;280(26):25291-7
The Journal of biological chemistry 2005 Jul 1;280(26):25291-7
Nicotine activates nuclear factor of activated T cells c2 (NFATc2) and prevents cell cycle entry in T cells.
Frazer-Abel AA, Baksh S, Fosmire SP, Willis D, Pierce AM, Meylemans H, Linthicum DS, Burakoff SJ, Coons T, Bellgrau D, Modiano JF
The Journal of pharmacology and experimental therapeutics 2004 Nov;311(2):758-69
The Journal of pharmacology and experimental therapeutics 2004 Nov;311(2):758-69
Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis.
Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, Aird WC
The Journal of biological chemistry 2004 Nov 26;279(48):50537-54
The Journal of biological chemistry 2004 Nov 26;279(48):50537-54
Interleukin (IL)-15 and IL-2 reciprocally regulate expression of the chemokine receptor CX3CR1 through selective NFAT1- and NFAT2-dependent mechanisms.
Barlic J, McDermott DH, Merrell MN, Gonzales J, Via LE, Murphy PM
The Journal of biological chemistry 2004 Nov 19;279(47):48520-34
The Journal of biological chemistry 2004 Nov 19;279(47):48520-34
TRPC3 channels confer cellular memory of recent neuromuscular activity.
Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS
Proceedings of the National Academy of Sciences of the United States of America 2004 Jun 22;101(25):9387-92
Proceedings of the National Academy of Sciences of the United States of America 2004 Jun 22;101(25):9387-92
Regulation of the murine Nfatc1 gene by NFATc2.
Zhou B, Cron RQ, Wu B, Genin A, Wang Z, Liu S, Robson P, Baldwin HS
The Journal of biological chemistry 2002 Mar 22;277(12):10704-11
The Journal of biological chemistry 2002 Mar 22;277(12):10704-11
A T cell-specific enhancer of the human CD40 ligand gene.
Schubert LA, Cron RQ, Cleary AM, Brunner M, Song A, Lu LS, Jullien P, Krensky AM, Lewis DB
The Journal of biological chemistry 2002 Mar 1;277(9):7386-95
The Journal of biological chemistry 2002 Mar 1;277(9):7386-95
The basis for TCR-mediated regulation of the IL-2 receptor alpha chain gene: role of widely separated regulatory elements.
Kim HP, Leonard WJ
The EMBO journal 2002 Jun 17;21(12):3051-9
The EMBO journal 2002 Jun 17;21(12):3051-9
Double-stranded RNA regulates IL-4 expression.
Kehoe KE, Brown MA, Imani F
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Double-stranded RNA regulates IL-4 expression.
Kehoe KE, Brown MA, Imani F
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Journal of immunology (Baltimore, Md. : 1950) 2001 Sep 1;167(5):2496-501
Identification and characterization of a novel nuclear factor of activated T-cells-1 isoform expressed in mouse brain.
Plyte S, Boncristiano M, Fattori E, Galvagni F, Paccani SR, Majolini MB, Oliviero S, Ciliberto G, Telford JL, Baldari CT
The Journal of biological chemistry 2001 Apr 27;276(17):14350-8
The Journal of biological chemistry 2001 Apr 27;276(17):14350-8
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, Kandarian SC
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
The calcineurin-NFAT pathway and muscle fiber-type gene expression.
Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, Kandarian SC
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
American journal of physiology. Cell physiology 2000 Oct;279(4):C915-24
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Chow CW, Rincón M, Cavanagh J, Dickens M, Davis RJ
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway.
Chow CW, Rincón M, Cavanagh J, Dickens M, Davis RJ
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Science (New York, N.Y.) 1997 Nov 28;278(5343):1638-41
Expression of the transcription factor NFATp in a neuronal cell line and in the murine nervous system.
Ho AM, Jain J, Rao A, Hogan PG
The Journal of biological chemistry 1994 Nov 11;269(45):28181-6
The Journal of biological chemistry 1994 Nov 11;269(45):28181-6
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
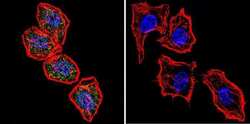
- Experimental details
- Immunofluorescent analysis of NFATc2 using NFATc2 Monoclonal Antibody (25A10.D6.D2) (Product # MA1-025) shows staining in Hela Cells. NFATc2 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc2 (Product # MA1-025) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
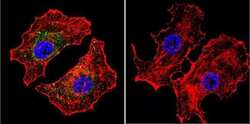
- Experimental details
- Immunofluorescent analysis of NFATc2 using NFATc2 Monoclonal Antibody (25A10.D6.D2) (Product # MA1-025) shows staining in MCF-7 Cells. NFATc2 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc2 (Product # MA1-025) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
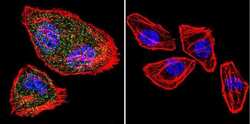
- Experimental details
- Immunofluorescent analysis of NFATc2 using NFATc2 Monoclonal Antibody (25A10.D6.D2) (Product # MA1-025) shows staining in U251 Cells. NFATc2 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc2 (Product # MA1-025) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
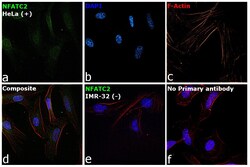
- Experimental details
- Immunofluorescence analysis of NFATC2 Monoclonal Antibody (25A10.D6.D2) was performed using 70% confluent log phase HeLa and IMR32 cells. The cells were fixed with 4% Paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 10 minutes at room temperature. The cells were labeled with NFATC2 Monoclonal Antibody (Product # MA1-025) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175, 1:2000 dilution) for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear and cytoplasmic localization. Panel e represents IMR-32 cells having no expression of NFATC2. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
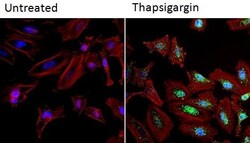
- Experimental details
- Immunofluorescent analysis of NFATc2 (green) in HeLa cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were left untreated (left panel) or treated with 1uM staurosporine (right panel) for 3 hours and probed with a NFATc2 monoclonal antibody (Product # MA1-025), at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with DyLight 554 Phalloidin (Product # 21834) and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan and ToxInsight at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
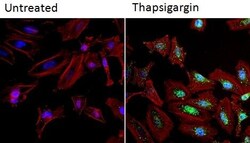
- Experimental details
- Immunofluorescent analysis of NFATc2 (green) in HeLa cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were left untreated (left panel) or treated with 1uM staurosporine (right panel) for 3 hours and probed with a NFATc2 monoclonal antibody (Product # MA1-025), at a dilution of 1:100 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with DyLight 554 Phalloidin (Product # 21834) and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan and ToxInsight at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
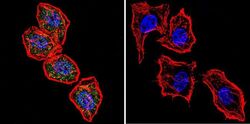
- Experimental details
- Immunofluorescent analysis of NFATc2 using NFATc2 Monoclonal Antibody (25A10.D6.D2) (Product # MA1-025) shows staining in Hela Cells. NFATc2 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc2 (Product # MA1-025) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
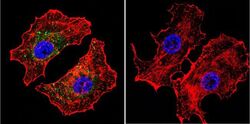
- Experimental details
- Immunofluorescent analysis of NFATc2 using NFATc2 Monoclonal Antibody (25A10.D6.D2) (Product # MA1-025) shows staining in MCF-7 Cells. NFATc2 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc2 (Product # MA1-025) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
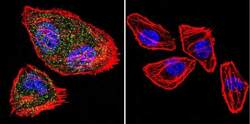
- Experimental details
- Immunofluorescent analysis of NFATc2 using NFATc2 Monoclonal Antibody (25A10.D6.D2) (Product # MA1-025) shows staining in U251 Cells. NFATc2 (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing NFATc2 (Product # MA1-025) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
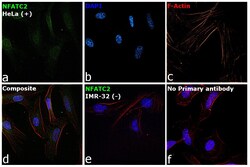
- Experimental details
- Immunofluorescence analysis of NFATC2 Monoclonal Antibody (25A10.D6.D2) was performed using 70% confluent log phase HeLa and IMR32 cells. The cells were fixed with 4% Paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 10 minutes at room temperature. The cells were labeled with NFATC2 Monoclonal Antibody (Product # MA1-025) at 1:100 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175, 1:2000 dilution) for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing nuclear and cytoplasmic localization. Panel e represents IMR-32 cells having no expression of NFATC2. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
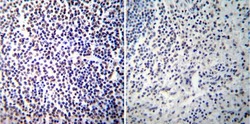
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human spleen tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing NFATc2 (Product # MA1-025) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
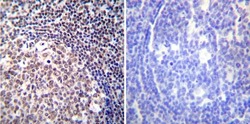
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human tonsil tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing NFATc2 (Product # MA1-025) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
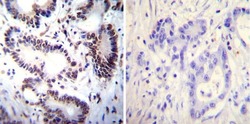
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human colon carcinoma tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing NFATc2 (Product # MA1-025) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
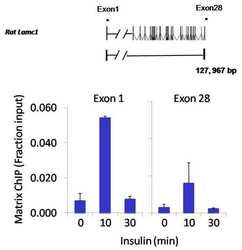
- Chromatin immunoprecipitation analysis of NFATc2 was performed using cross-linked chromatin from 1x10^6 HTC-IR rat hepatoma cells treated with insulin for 0, 10, and 30 minutes. Immunoprecipitation was performed using a multiplex microplate Matrix ChIP assay (see reference for Matrix ChIP protocol: http://www.ncbi.nlm.nih.gov/pubmed/22098709) with 1.0 µL/100 µL well volume of an NFATc2 monoclonal antibody (Product # MA1-025). Chromatin aliquots from ~1x10^5 cells were used per ChIP pull-down. Quantitative PCR data were done in quadruplicate using 1 µL of eluted DNA in 2 µL SYBR real-time PCR reactions containing primers to amplify exon-1 or exon-28 of the LAMC1 gene. PCR calibration curves were generated for each primer pair from a dilution series of sheared total genomic DNA. Quantitation of immunoprecipitated chromatin is presented as signal relative to the total amount of input chromatin. Results represent the mean +/- SEM for three experiments. A schematic representation of the rat LAMC1 locus is shown above the data where boxes represent exons (black boxes = translated regions, white boxes = untranslated regions), the zigzag line represents an intron, and the straight line represents upstream sequence. Regions amplified by LAMC1 primers are represented by black bars. Data courtesy of the Innovators Program.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
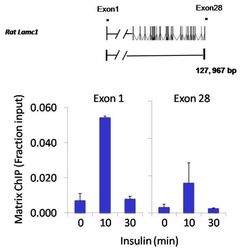
- Chromatin immunoprecipitation analysis of NFATc2 was performed using cross-linked chromatin from 1x10^6 HTC-IR rat hepatoma cells treated with insulin for 0, 10, and 30 minutes. Immunoprecipitation was performed using a multiplex microplate Matrix ChIP assay (see reference for Matrix ChIP protocol: http://www.ncbi.nlm.nih.gov/pubmed/22098709) with 1.0 µL/100 µL well volume of an NFATc2 monoclonal antibody (Product # MA1-025). Chromatin aliquots from ~1x10^5 cells were used per ChIP pull-down. Quantitative PCR data were done in quadruplicate using 1 µL of eluted DNA in 2 µL SYBR real-time PCR reactions containing primers to amplify exon-1 or exon-28 of the LAMC1 gene. PCR calibration curves were generated for each primer pair from a dilution series of sheared total genomic DNA. Quantitation of immunoprecipitated chromatin is presented as signal relative to the total amount of input chromatin. Results represent the mean +/- SEM for three experiments. A schematic representation of the rat LAMC1 locus is shown above the data where boxes represent exons (black boxes = translated regions, white boxes = untranslated regions), the zigzag line represents an intron, and the straight line represents upstream sequence. Regions amplified by LAMC1 primers are represented by black bars. Data courtesy of the Innovators Program.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
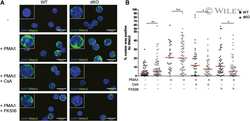
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
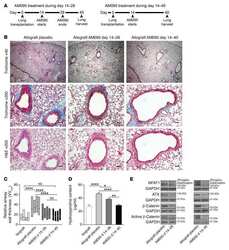
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
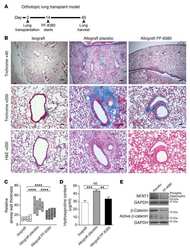
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
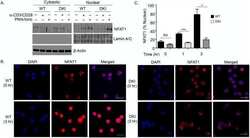
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
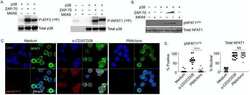
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
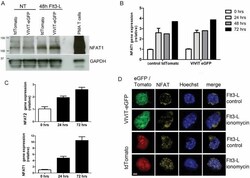
- Experimental details
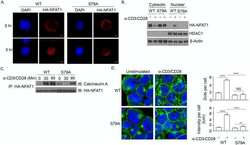
- Figure 1 NFAT1 is expressed in HSC lines and increases following stimulation with Flt3-L Western blot analysis of un-stimulated and Flt3-L stimulated VIVIT expressing and control HSC lines. PMA treated splenic T cells were used as a positive control. qPCR analysis of NFAT1 mRNA expression in HSC lines after stimulation with Flt3-L. qPCR analysis of NFAT2 and NFAT1 expression in freshly isolated lineage (lin - ) negative cells from BM stimulated with Flt3-L. Confocal images of VIVIT-eGFP and control-tdTomato expressing HSC lines stimulated for 48 h with Flt3-L. NFAT translocation was induced by treatment with ionomycin for 2 h (original magnification, x200, scale bar 2 um). Data are representative of three ( A and C ) and five ( B ) independent experiments (mean +- SEM). Three mice per group for each time-point were used ( C ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
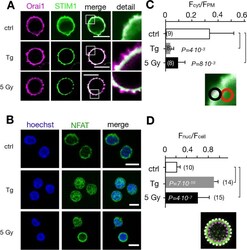
- Experimental details
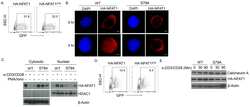
- Figure 5. Calcium-dependent SOCE/NFAT pathway is activated by IR in naive T-lymphocytes. (A) Distribution of endogenous Orai1 (magenta, first column) and STIM1 (green, second column) in fixed PBMCs immunostained with secondary antibodies Alx488 and Alx647, respectively. A merge of the two channels is shown in the third column, with higher magnification of marked areas in the fourth column. The merge of untreated control cells additionally shows the nucleus stained with Hoechst DNA dye (blue). Images show cells that were fixed as untreated/nonirradiated control cells (ctrl, top row) and cells fixed 15 min after treatment with 2 uM Tg (second row) or after exposing cells to 5 Gy (third row). (B) Mean ratio (+- SD, number of cells in brackets) of green fluorescence in ROI (inset image, red circle) in cytosol divided by fluorescence in ROI in direct vicinity over PM (black circle). Data from untreated control cells (ctrl) as well as cells exposed to 2 muM Tg or 5 Gy x rays 60 min after treatment. (C) Confocal images of PBMCs showing nucleus stained with Hoechst DNA dye (blue, first column) and endogenous NFATc2 (green, second column) stained with Alx488. Overlay of both columns is shown in third column. Cells were fixed immediately (untreated/nonirradiated control, top row), 15 min after 2 uM Tg Ca 2+ store depletion (second row) or 60 min after x-ray exposure with 5 Gy (third/fourth row). All scale bars, 10 mum. (D) Mean ratio (+- SD, number of cells in brackets) of GFP fluoresc
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
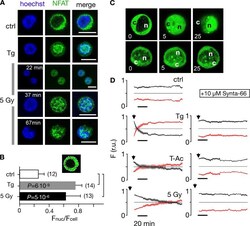
- Experimental details
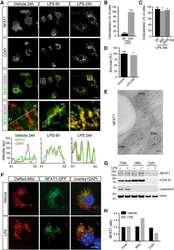
- Figure 7. Nuclear translocation of Ca 2+ -dependent NFAT in Jurkat cells. (A) Confocal images of Jurkat cells in which nucleus was stained with Hoechst DNA dye (blue, first column) and endogenous NFATc2 immunostained with secondary antibody Alx488 (green, second column). The third column shows a merge of blue and green channels. Cells were fixed immediately in untreated/nonirradiated control cells (ctrl, first row), 15 min after 2 uM Tg Ca 2+ store depletion (Tg, second row), or 15, 30, and 60 min after x-ray exposure with 5 Gy (three bottom rows). (B) Mean ratio (+- SD, number of cells in brackets) of GFP fluorescence in nucleus (inset, magenta circle) divided by fluorescence of total cell (white circle). Statistical differences between treatments were analyzed by unpaired Student's t test, and respective P values are given in the figure. (C) Live-cell imaging of nuclear import of transiently expressed NFATc2-GFP from cytosol (c) to nucleus (n) in Jurkat cells after stimulation with 2 muM Tg in absence (top) or presence (bottom) of 10 uM CRAC channel inhibitor Synta66. Numbers indicate time in minutes after treatment. (D) Kinetic analysis of NFATc2-GFP nuclear translocation from cytosol (black) to nucleus (red). Data are from confocal imaging of Jurkat cells in untreated control condition (crtl), with 2 uM Tg in 25 mul/ml activator (T-Ac), or after 5 Gy x-ray exposure (5 Gy). Data were obtained without (left) and with (right) 10 uM Synta66. Each time course diagram is the me
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
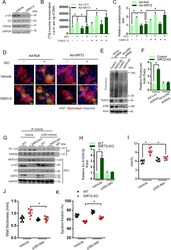
- Experimental details
- Figure 5. GSK3beta is required for the anti-hypertrophic role of SIRT2 deacetylase. ( A ) Western blotting analysis depicting the activity of GSK3 inhibitor X (GSK3-X). Neonatal rat cardiomyocytes were treated with vehicle or 500 nM GSK3-X for 48 hr and the activity of GSK3 was assessed by monitoring the phosphorylation of GS by specific antibody. ( B ) [ 3 H]-leucine incorporation into total cellular protein of control (Ad-GFP) or SIRT2-overexpressing (Ad-SIRT2) rat neonatal cardiomyocytes treated with either vehicle or 500 nM GSK3 inhibitor X (GSK3-X) for 48 hr. Cardiomyocytes were infected with adenoviral vectors encoding either GFP or SIRT2 for 24 hr prior to GSK3-X treatment. After the GSK3-X treatment, cardiomyocytes were stimulated with either vehicle or 20 uM ISO for 24 hr and the [ 3 H]-leucine incorporation was monitored. c.p.m. counts per minute. n = 10. Data is presented as mean +- s.d. *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
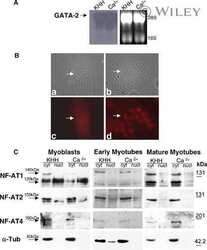
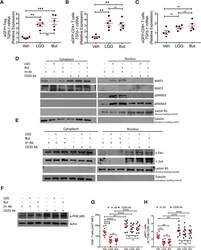
- Figure 7. Effects of LGG and Butyrate (But) on TGFb1 Production by BM T Cells, onNFAT1/2 and SMAD2/3 Activation, and on PI3K and Akt Signaling in BMCD8 + T Cells (A-C) TGFb1 mRNA expression by FACS-sorted BM conventionaleGFPCD4 + T cells, eGFPCD8 + T cells, andeGFP + Treg cells. DEREG (eGFP.Foxp3) reporter mice were treatedwith vehicle, LGG, or butyrate for 4 weeks. BM cells were sorted at the end ofthe treatment period. (D) Immunoblot analysis for the detection of NFAT1, NFAT2, pSMAD2, andpSMAD3 in purified BM CD8 + T cells. (E) Immunoblot analysis for the detection of c-Jun and c-Fos inpurified BM CD8 + T cells. (D and E) Fresh BM CD8 + T cells were pooled together andthen used for obtaining nuclear and cytoplasmic fractions. Laminin B1 was usedas nuclear loading control. Tubulin was used as cytoplasmic loading control. (F) Immunoblot analysis for the detection of Phospho-PI3K p85 in thewhole lysate from BM CD8 + T cells. (G and H) pAKT levels in BM CD8 + T cells and percent ofpAKT + BM CD8 + T cells, as determined by flowcytometry. (D-F) Conventionally raised WT mice were treated with vehicle,LGG, or butyrate for 4 weeks. BM CD8 + T cells were purified at theend of the treatment period using the EasySep Mouse CD8 + T CellIsolation Kit. One representative experiment of 3 experiments. Data were expressed as mean +- SEM, n = 5-10 mice pergroup in (A)-(C), (G), and (H). Data were analyzed by two-way ANOVA andpost hoc tests applying the Bonferroni correction for multiple compar
 Explore
Explore Validate
Validate Learn
Learn