Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunohistochemistry [2]
- Chromatin Immunoprecipitation [2]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-40839 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- MED17 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Peptide sequence: AAQILLKGAE RLTKSVTENQ ENKLQRDFNS ELLRLRQHWK LRKVGDKILG Sequence homology: Cow: 100%; Dog: 100%; Guinea Pig: 100%; Horse: 100%; Human: 100%; Mouse: 92%; Rabbit: 100%; Rat: 100%
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 0.5 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references The oncogenicity of tumor-derived mutant p53 is enhanced by the recruitment of PLK3.
Vaughan CA, Singh S, Subler MA, Windle JJ, Inoue K, Fry EA, Pillappa R, Grossman SR, Windle B, Andrew Yeudall W, Deb SP, Deb S
Nature communications 2021 Jan 29;12(1):704
Nature communications 2021 Jan 29;12(1):704
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
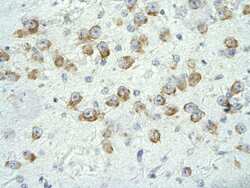
- Experimental details
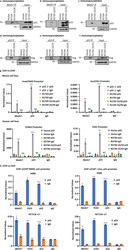
- Immunohistochemistry (paraffin-embedded) analysis of human neural tissue using an anti-CRSP6 polyclonal antibody (Product # PA5-40839).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
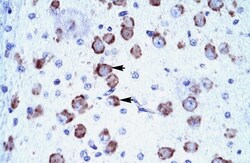
- Experimental details
- Immunohistochemistry (paraffin-embedded) analysis of human brain cells using an anti-CRSP6 polyclonal antibody (Product # PA5-40839).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
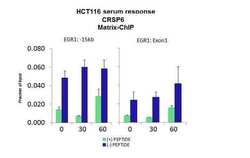
- Experimental details
- ChIP assay analysis of quiescent human colon carcinoma HCT116 cells using an anti-CRSP6 polyclonal antibody (Product # PA5-40839).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
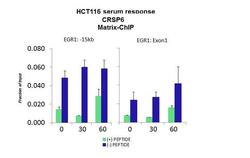
- Experimental details
- ChIP assay analysis of quiescent human colon carcinoma HCT116 cells using an anti-CRSP6 polyclonal antibody (Product # PA5-40839).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
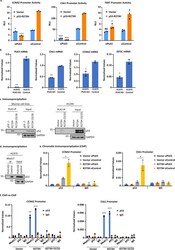
- Fig. 4 GOF p53 interacts with Med17 and amino acid acids at mouse p53 L25, W26 (L22, W23 in human) are important for this interaction. a Immunoprecipitation experiment showing complex formation between murine p53-R172H and the Mediator protein 17 (Med17) in solution. Nuclear extracts were made from murine lung cells expressing p53-R172H (generated by adeno Cre as described in the text), p53-L25Q/W26S/R172H, and control, and immunoprecipitated by Med17 antibody and probed with anti-p53 antibody. b Immunoprecipitation experiment showing complex formation between p53-R273H with Med17 in solution. Nuclear extracts were prepared from H1299 cells stably transfected with empty vector, expressing p53-R273H and -L22Q/W23S/R273H, and immunoprecipitated by Med17 antibody and probed with anti-p53 antibody. c Immunoprecipitation experiment showing complex formation between p53-R175H and Med17 in solution. Nuclear extracts were prepared from H1299 cells stably transfected with empty vector, expressing p53-R175H and -L22Q/W23S/R175H, and immunoprecipitated by Med17 antibody and probed with anti-p53 antibody. d Reverse immunoprecipitation experiment showing complex formation between murine p53-R172H and the Mediator protein 17 (Med17) in solution. Whole cell extracts were made from murine lung cells expressing p53-R172H (generated by adeno Cre as described in the text), p53-L25Q/W26S/R172H, and control after transfection with Flag-Med17, and immunoprecipitated by p53 antibody and probed with
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 8 PLK3 controls GOF p53's transactivation and binding to its inducible gene promoters. a Transient transactivation assay of H1299 cells transfected with the p53-R273H (or empty vector) along with siRNA against PLK3 (or scrambled control) and the cyclin A promoter, Chk1 promoter, or the TERT promoter-luciferase construct. Transient transcriptional assays were carried out in H1299 cells. Cells were harvested after 24 h of transfection. Firefly luciferase readings were plotted as relative light units (RLU) and is presented as the mean +- SEM ( n = 3, performed twice). A two-sided Student's t -test was performed and CCNA2 * p = 0.01, *** p = 6.5E-7; Chk1 *** p < 2.5E-6; TERT * p = 0.01. b H1975 PLK3 KO cells were used to determine the ability of GOF p53 to upregulate its activated genes. Transcript levels of PLK3, Chk1, CCNA2, and EIF3C were measured by QPCR. Data are normalized with GAPDH and are presented as the mean +- SEM ( n = 3). A two-sided Student's t -test was performed and PLK3 ** p = 0.002; Chk1 ** p = 0.001; CCNA2 * p = 0.03; EIF3C * p = 0.02. c Co-immunoprecipitation of mutant p53 and PLK3 in murine and human cells. Mouse R172H and human R273H were able to interact with PLK3 but their transactivation-deficient counterparts, L25Q/W26S/R172H and L22Q/W23S/R273H were not. d PLK3 knockout (KO) cells were used to determine whether the interaction between mutant p53 and Med17 has any requirement for PLK3. Co-immunoprecipitation in H1975 PLK3 KO cells versus control sh
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry