Antibody data
- Antibody Data
- Antigen structure
- References [14]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [1]
- Immunohistochemistry [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- HPA035240 - Provider product page

- Provider
- Atlas Antibodies
- Proper citation
- Atlas Antibodies Cat#HPA035240, RRID:AB_10604092
- Product name
- Anti-SLC1A5
- Antibody type
- Polyclonal
- Description
- Polyclonal Antibody against Human SLC1A5, Gene description: solute carrier family 1 (neutral amino acid transporter), member 5, Alternative Gene Names: AAAT, ASCT2, M7V1, RDRC, Validated applications: WB, IHC, ICC, Uniprot ID: Q15758, Storage: Store at +4°C for short term storage. Long time storage is recommended at -20°C.
- Reactivity
- Human
- Host
- Rabbit
- Conjugate
- Unconjugated
- Isotype
- IgG
- Vial size
- 100 µl
- Storage
- Store at +4°C for short term storage. Long time storage is recommended at -20°C.
- Handling
- The antibody solution should be gently mixed before use.
Submitted references Combinatorial targeting of glutamine metabolism and lysosomal-based lipid metabolism effectively suppresses glioblastoma
Macropinocytosis mediates resistance to loss of glutamine transport in triple-negative breast cancer
Receptor usage of Syncytin-1: ASCT2, but not ASCT1, is a functional receptor and effector of cell fusion in the human placenta
Chorioallantoic membrane assay revealed the role of TIPARP (2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible poly (ADP-ribose) polymerase) in lung adenocarcinoma-induced angiogenesis
SLC1A5 co-expression with TALDO1 associates with endocrine therapy failure in estrogen receptor-positive breast cancer
Integrated Metabolic and Epigenomic Reprograming by H3K27M Mutations in Diffuse Intrinsic Pontine Gliomas
EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and c-MYC
TFEB controls retromer expression in response to nutrient availability
Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma.
The GSK3 Signaling Axis Regulates Adaptive Glutamine Metabolism in Lung Squamous Cell Carcinoma
Epigenetic silencing of microRNA-137 enhances ASCT2 expression and tumor glutamine metabolism
Non-Invasive Glutamine PET Reflects Pharmacological Inhibition of BRAFV600E In Vivo
Regulation of Glutamine Carrier Proteins by RNF5 Determines Breast Cancer Response to ER Stress-Inducing Chemotherapies
Targeting ASCT2‐mediated glutamine uptake blocks prostate cancer growth and tumour development
Zhong Y, Geng F, Mazik L, Yin X, Becker A, Mohammed S, Su H, Xing E, Kou Y, Chiang C, Fan Y, Guo Y, Wang Q, Li P, Mo X, Lefai E, He L, Cheng X, Zhang X, Chakravarti A, Guo D
Cell Reports Medicine 2024;5(9):101706
Cell Reports Medicine 2024;5(9):101706
Macropinocytosis mediates resistance to loss of glutamine transport in triple-negative breast cancer
Wahi K, Freidman N, Wang Q, Devadason M, Quek L, Pang A, Lloyd L, Larance M, Zanini F, Harvey K, O’Toole S, Guan Y, Holst J
The EMBO Journal 2024;43(23):5857-5882
The EMBO Journal 2024;43(23):5857-5882
Receptor usage of Syncytin-1: ASCT2, but not ASCT1, is a functional receptor and effector of cell fusion in the human placenta
Štafl K, Trávníček M, Janovská A, Kučerová D, Pecnová Ľ, Yang Z, Stepanec V, Jech L, Salker M, Hejnar J, Trejbalová K
Proceedings of the National Academy of Sciences 2024;121(44)
Proceedings of the National Academy of Sciences 2024;121(44)
Chorioallantoic membrane assay revealed the role of TIPARP (2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible poly (ADP-ribose) polymerase) in lung adenocarcinoma-induced angiogenesis
Miura K, Koyanagi-Aoi M, Maniwa Y, Aoi T
Cancer Cell International 2023;23(1)
Cancer Cell International 2023;23(1)
SLC1A5 co-expression with TALDO1 associates with endocrine therapy failure in estrogen receptor-positive breast cancer
Alfarsi L, El Ansari R, Craze M, Mohammed O, Masisi B, Ellis I, Rakha E, Green A
Breast Cancer Research and Treatment 2021;189(2):317-331
Breast Cancer Research and Treatment 2021;189(2):317-331
Integrated Metabolic and Epigenomic Reprograming by H3K27M Mutations in Diffuse Intrinsic Pontine Gliomas
Chung C, Sweha S, Pratt D, Tamrazi B, Panwalkar P, Banda A, Bayliss J, Hawes D, Yang F, Lee H, Shan M, Cieslik M, Qin T, Werner C, Wahl D, Lyssiotis C, Bian Z, Shotwell J, Yadav V, Koschmann C, Chinnaiyan A, Blüml S, Judkins A, Venneti S
Cancer Cell 2020;38(3):334-349.e9
Cancer Cell 2020;38(3):334-349.e9
EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and c-MYC
Krishnamoorthy G, Davidson N, Leach S, Zhao Z, Lowe S, Lee G, Landa I, Nagarajah J, Saqcena M, Singh K, Wendel H, Dogan S, Tamarapu P, Blenis J, Ghossein R, Knauf J, Rätsch G, Fagin J
Cancer Discovery 2019;9(2):264-281
Cancer Discovery 2019;9(2):264-281
TFEB controls retromer expression in response to nutrient availability
Curnock R, Calcagni A, Ballabio A, Cullen P
Journal of Cell Biology 2019;218(12):3954-3966
Journal of Cell Biology 2019;218(12):3954-3966
Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma.
Okazaki S, Umene K, Yamasaki J, Suina K, Otsuki Y, Yoshikawa M, Minami Y, Masuko T, Kawaguchi S, Nakayama H, Banno K, Aoki D, Saya H, Nagano O
Cancer science 2019 Nov;110(11):3453-3463
Cancer science 2019 Nov;110(11):3453-3463
The GSK3 Signaling Axis Regulates Adaptive Glutamine Metabolism in Lung Squamous Cell Carcinoma
Momcilovic M, Bailey S, Lee J, Fishbein M, Braas D, Go J, Graeber T, Parlati F, Demo S, Li R, Walser T, Gricowski M, Shuman R, Ibarra J, Fridman D, Phelps M, Badran K, St. John M, Bernthal N, Federman N, Yanagawa J, Dubinett S, Sadeghi S, Christofk H, Shackelford D
Cancer Cell 2018;33(5):905-921.e5
Cancer Cell 2018;33(5):905-921.e5
Epigenetic silencing of microRNA-137 enhances ASCT2 expression and tumor glutamine metabolism
Dong J, Xiao D, Zhao Z, Ren P, Li C, Hu Y, Shi J, Su H, Wang L, Liu H, Li B, Gao P, Qing G
Oncogenesis 2017;6(7):e356-e356
Oncogenesis 2017;6(7):e356-e356
Non-Invasive Glutamine PET Reflects Pharmacological Inhibition of BRAFV600E In Vivo
Schulte M, Hight M, Ayers G, Liu Q, Shyr Y, Washington M, Manning H
Molecular Imaging and Biology 2016;19(3):421-428
Molecular Imaging and Biology 2016;19(3):421-428
Regulation of Glutamine Carrier Proteins by RNF5 Determines Breast Cancer Response to ER Stress-Inducing Chemotherapies
Jeon Y, Khelifa S, Ratnikov B, Scott D, Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill L, Jiang T, Rimm D, Cardiff R, Mills G, Smith J, Osterman A, Kluger Y, Ronai Z
Cancer Cell 2015;27(3):354-369
Cancer Cell 2015;27(3):354-369
Targeting ASCT2‐mediated glutamine uptake blocks prostate cancer growth and tumour development
Wang Q, Hardie R, Hoy A, van Geldermalsen M, Gao D, Fazli L, Sadowski M, Balaban S, Schreuder M, Nagarajah R, Wong J, Metierre C, Pinello N, Otte N, Lehman M, Gleave M, Nelson C, Bailey C, Ritchie W, Rasko J, Holst J
The Journal of Pathology 2015;236(3):278-289
The Journal of Pathology 2015;236(3):278-289
No comments: Submit comment
Enhanced validation
- Submitted by
- Atlas Antibodies (provider)
- Enhanced method
- Recombinant expression validation
- Main image
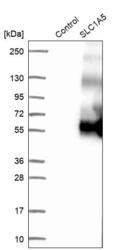
- Experimental details
- Western blot analysis in control (vector only transfected HEK293T lysate) and SLC1A5 over-expression lysate (Co-expressed with a C-terminal myc-DDK tag (~3.1 kDa) in mammalian HEK293T cells, LY417160).
- Sample type
- Human
- Protocol
- Protocol
Supportive validation
- Submitted by
- Atlas Antibodies (provider)
- Main image
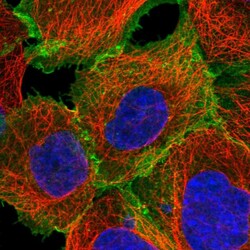
- Experimental details
- Immunofluorescent staining of human cell line A-431 shows localization to plasma membrane.
- Sample type
- Human
Supportive validation
- Submitted by
- Atlas Antibodies (provider)
- Enhanced method
- Orthogonal validation
- Main image
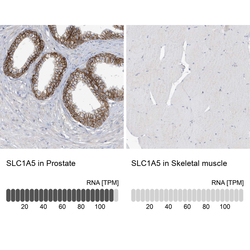
- Experimental details
- Immunohistochemistry analysis in human prostate and skeletal muscle tissues using HPA035240 antibody. Corresponding SLC1A5 RNA-seq data are presented for the same tissues.
- Sample type
- Human
- Protocol
- Protocol
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry