Antibody data
- Antibody Data
- Antigen structure
- References [100]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [1]
- Immunoprecipitation [1]
- Immunohistochemistry [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 60019-2-Ig - Provider product page

- Provider
- Proteintech Group
- Proper citation
- Proteintech Cat#60019-2-Ig, RRID:AB_2200520
- Product name
- TDP-43 (human specific) antibody
- Antibody type
- Monoclonal
- Description
- TDP-43 (human specific) antibody (Cat. #60019-2-Ig) is a mouse monoclonal antibody that shows reactivity with human and has been validated for the following applications: FC, IHC, IP, WB, ELISA.
- Reactivity
- Human
- Host
- Mouse
- Conjugate
- Unconjugated
- Isotype
- IgG
- Antibody clone number
- 6H6E12
- Vial size
- 20ul, 150ul
Submitted references A Panel of miRNA Biomarkers Common to Serum and Brain-Derived Extracellular Vesicles Identified in Mouse Model of Amyotrophic Lateral Sclerosis.
Targeting 14-3-3θ-mediated TDP-43 pathology in amyotrophic lateral sclerosis and frontotemporal dementia mice.
Loss of TDP-43 oligomerization or RNA binding elicits distinct aggregation patterns.
Dysregulation of the progranulin-driven autophagy-lysosomal pathway mediates secretion of the nuclear protein TDP-43.
Tandem detergent-extraction and immunoprecipitation of proteinopathy: Scalable enrichment of ALS-associated TDP-43 aggregates.
Sustained therapeutic benefits by transient reduction of TDP-43 using ENA-modified antisense oligonucleotides in ALS/FTD mice.
Regulation of RNG105/caprin1 dynamics by pathogenic cytoplasmic FUS and TDP-43 in neuronal RNA granules modulates synaptic loss.
Pathologically mislocalised TDP-43 in upper motor neurons causes a die-forward spread of ALS-like pathogenic changes throughout the mouse corticomotor system.
Atypical TDP-43 protein expression in an ALS pedigree carrying a p.Y374X truncation mutation in TARDBP.
Pathological Comparison of TDP-43 Between Motor Neurons and Interneurons Expressed by a Tetracycline Repressor System on the Mouse Artificial Chromosome.
Distinctive chaperonopathy in skeletal muscle associated with the dominant variant in DNAJB4.
NEMO reshapes the α-Synuclein aggregate interface and acts as an autophagy adapter by co-condensation with p62.
Targeting RACK1 to alleviate TDP-43 and FUS proteinopathy-mediated suppression of protein translation and neurodegeneration.
TDP-43 pathology and functional deficits in wild-type and ALS/FTD mutant cyclin F mouse models.
Astrocytic TDP-43 dysregulation impairs memory by modulating antiviral pathways and interferon-inducible chemokines.
Extremely Early-Onset Frontotemporal Dementia: A Case Report and Literature Review.
Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43.
Estrogen Enhances Dendrite Spine Function and Recovers Deficits in Neuroplasticity in the prpTDP-43(A315T) Mouse Model of Amyotrophic Lateral Sclerosis.
A single-cell atlas of human and mouse white adipose tissue.
Frontotemporal Lobar Degeneration Case with an N-Terminal TUBA4A Mutation Exhibits Reduced TUBA4A Levels in the Brain and TDP-43 Pathology.
A quantitative biology approach correlates neuronal toxicity with the largest inclusions of TDP-43.
TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration.
miR-23a suppression accelerates functional decline in the rNLS8 mouse model of TDP-43 proteinopathy.
Detection of Pathological Markers of Neurodegenerative Diseases following Microfluidic Direct Conversion of Patient Fibroblasts into Neurons.
Pathological changes induced by Alzheimer's brain inoculation in amyloid-beta plaque-bearing mice.
Disease-linked TDP-43 hyperphosphorylation suppresses TDP-43 condensation and aggregation.
Hsp90 and its co-chaperone Sti1 control TDP-43 misfolding and toxicity.
CdSe Quantum Dots in Human Models Derived from ALS Patients: Characterization, Nuclear Penetration Studies and Multiplexing.
TDP-43 and PINK1 mediate CHCHD10(S59L) mutation-induced defects in Drosophila and in vitro.
Cytoplasmic Human TDP-43 Mislocalization Induces Widespread Dendritic Spine Loss in Mouse Upper Motor Neurons.
Increasing Brain Permeability of PHA-767491, a Cell Division Cycle 7 Kinase Inhibitor, with Biodegradable Polymeric Nanoparticles.
Spreading of TDP-43 pathology via pyramidal tract induces ALS-like phenotypes in TDP-43 transgenic mice.
Generation and analysis of innovative genomically humanized knockin SOD1, TARDBP (TDP-43), and FUS mouse models.
Stress Granule Assembly Can Facilitate but Is Not Required for TDP-43 Cytoplasmic Aggregation.
ALS skin fibroblasts reveal oxidative stress and ERK1/2-mediated cytoplasmic localization of TDP-43.
RBM45 associates with nuclear stress bodies and forms nuclear inclusions during chronic cellular stress and in neurodegenerative diseases.
Homozygous GRN mutations: new phenotypes and new insights into pathological and molecular mechanisms.
Neurodegeneration and Motor Deficits in the Absence of Astrogliosis upon Transgenic Mutant TDP-43 Expression in Mature Mice.
Leukocyte Derived Microvesicles as Disease Progression Biomarkers in Slow Progressing Amyotrophic Lateral Sclerosis Patients.
Multiple System Atrophy With Predominant Striatonigral Degeneration and TAR DNA-Binding Protein of 43 kDa Pathology: An Unusual Variant of Multiple System Atrophy.
Amyotrophic lateral sclerosis mutant TDP-43 may cause synaptic dysfunction through altered dendritic spine function.
Phosphorylated and aggregated TDP-43 with seeding properties are induced upon mutant Huntingtin (mHtt) polyglutamine expression in human cellular models.
TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates.
Single-copy expression of an amyotrophic lateral sclerosis-linked TDP-43 mutation (M337V) in BAC transgenic mice leads to altered stress granule dynamics and progressive motor dysfunction.
The role of lysosomes and autophagosomes in frontotemporal lobar degeneration.
mTh1 driven expression of hTDP-43 results in typical ALS/FTLD neuropathological symptoms.
Pathological Proteins Are Transported by Extracellular Vesicles of Sporadic Amyotrophic Lateral Sclerosis Patients.
A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing.
Frontotemporal dementia with trans-activation response DNA-binding protein 43 presenting with catatonic syndrome.
Unexpected similarities between C9ORF72 and sporadic forms of ALS/FTD suggest a common disease mechanism.
RNA and Protein Interactors with TDP-43 in Human Spinal-Cord Lysates in Amyotrophic Lateral Sclerosis.
Split GFP technologies to structurally characterize and quantify functional biomolecular interactions of FTD-related proteins.
Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes.
Synapse Dysfunction of Layer V Pyramidal Neurons Precedes Neurodegeneration in a Mouse Model of TDP-43 Proteinopathies.
ALS/FTLD: experimental models and reality.
Endocytosis regulates TDP-43 toxicity and turnover.
Early Cognitive/Social Deficits and Late Motor Phenotype in Conditional Wild-Type TDP-43 Transgenic Mice.
Mislocated FUS is sufficient for gain-of-toxic-function amyotrophic lateral sclerosis phenotypes in mice.
Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains.
Increased cytoplasmic TARDBP mRNA in affected spinal motor neurons in ALS caused by abnormal autoregulation of TDP-43.
In vitro prion-like behaviour of TDP-43 in ALS.
Nuclear bodies reorganize during myogenesis in vitro and are differentially disrupted by expression of FSHD-associated DUX4.
The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity.
Templated Aggregation of TAR DNA-binding Protein of 43 kDa (TDP-43) by Seeding with TDP-43 Peptide Fibrils.
Gain-of-function profilin 1 mutations linked to familial amyotrophic lateral sclerosis cause seed-dependent intracellular TDP-43 aggregation.
Short-term suppression of A315T mutant human TDP-43 expression improves functional deficits in a novel inducible transgenic mouse model of FTLD-TDP and ALS.
TARDBP pathogenic mutations increase cytoplasmic translocation of TDP-43 and cause reduction of endoplasmic reticulum Ca²⁺ signaling in motor neurons.
Evidence of a link between ubiquilin 2 and optineurin in amyotrophic lateral sclerosis.
RBM45 Modulates the Antioxidant Response in Amyotrophic Lateral Sclerosis through Interactions with KEAP1.
An acetylation switch controls TDP-43 function and aggregation propensity.
Expression of FSHD-related DUX4-FL alters proteostasis and induces TDP-43 aggregation.
Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase.
Clinicopathological features of the first Asian family having vocal cord and pharyngeal weakness with distal myopathy due to a MATR3 mutation.
TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies.
Reversible behavioral phenotypes in a conditional mouse model of TDP-43 proteinopathies.
Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation.
Prevention of intestinal obstruction reveals progressive neurodegeneration in mutant TDP-43 (A315T) mice.
An autopsy case of sporadic amyotrophic lateral sclerosis associated with the I113T SOD1 mutation.
Distinct pathways leading to TDP-43-induced cellular dysfunctions.
TDP-43, an ALS linked protein, regulates fat deposition and glucose homeostasis.
Cytosolic 5'-nucleotidase 1A autoimmunity in sporadic inclusion body myositis.
Ubiquilin-2 (UBQLN2) binds with high affinity to the C-terminal region of TDP-43 and modulates TDP-43 levels in H4 cells: characterization of inhibition by nucleic acids and 4-aminoquinolines.
Prion-like properties of pathological TDP-43 aggregates from diseased brains.
A unique mouse model for investigating the properties of amyotrophic lateral sclerosis-associated protein TDP-43, by in utero electroporation.
The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation.
Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination.
Molecular analysis and biochemical classification of TDP-43 proteinopathy.
Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking.
Epitope mapping of antibodies against TDP-43 and detection of protease-resistant fragments of pathological TDP-43 in amyotrophic lateral sclerosis and frontotemporal lobar degeneration.
Association of UBQLN1 mutation with Brown-Vialetto-Van Laere syndrome but not typical ALS.
Inclusion body myositis coexisting with hypertrophic cardiomyopathy: an autopsy study.
AMSH is required to degrade ubiquitinated proteins in the central nervous system.
Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice.
Dysfunction of the ubiquitin-proteasome system in the cerebellum of aging Ts65Dn mice.
Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia.
FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis.
Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43.
Caspase-cleaved TAR DNA-binding protein-43 is a major pathological finding in Alzheimer's disease.
TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system.
Vassileff N, Spiers JG, Lee JD, Woodruff TM, Ebrahimie E, Mohammadi Dehcheshmeh M, Hill AF, Cheng L
Molecular neurobiology 2024 Aug;61(8):5901-5915
Molecular neurobiology 2024 Aug;61(8):5901-5915
Targeting 14-3-3θ-mediated TDP-43 pathology in amyotrophic lateral sclerosis and frontotemporal dementia mice.
Ke YD, van Hummel A, Au C, Chan G, Lee WS, van der Hoven J, Przybyla M, Deng Y, Sabale M, Morey N, Bertz J, Feiten A, Ippati S, Stevens CH, Yang S, Gladbach A, Haass NK, Kril JJ, Blair IP, Delerue F, Ittner LM
Neuron 2024 Apr 17;112(8):1249-1264.e8
Neuron 2024 Apr 17;112(8):1249-1264.e8
Loss of TDP-43 oligomerization or RNA binding elicits distinct aggregation patterns.
Pérez-Berlanga M, Wiersma VI, Zbinden A, De Vos L, Wagner U, Foglieni C, Mallona I, Betz KM, Cléry A, Weber J, Guo Z, Rigort R, de Rossi P, Manglunia R, Tantardini E, Sahadevan S, Stach O, Hruska-Plochan M, Allain FH, Paganetti P, Polymenidou M
The EMBO journal 2023 Sep 4;42(17):e111719
The EMBO journal 2023 Sep 4;42(17):e111719
Dysregulation of the progranulin-driven autophagy-lysosomal pathway mediates secretion of the nuclear protein TDP-43.
Tanaka Y, Ito SI, Honma Y, Hasegawa M, Kametani F, Suzuki G, Kozuma L, Takeya K, Eto M
The Journal of biological chemistry 2023 Nov;299(11):105272
The Journal of biological chemistry 2023 Nov;299(11):105272
Tandem detergent-extraction and immunoprecipitation of proteinopathy: Scalable enrichment of ALS-associated TDP-43 aggregates.
Evangelista BA, Cahalan SR, Ragusa JV, Mordant A, Necarsulmer JC, Perna RJ, Ajit T, White K, Barker NK, Tian X, Cohen S, Meeker R, Herring LE, Cohen TJ
iScience 2023 May 19;26(5):106645
iScience 2023 May 19;26(5):106645
Sustained therapeutic benefits by transient reduction of TDP-43 using ENA-modified antisense oligonucleotides in ALS/FTD mice.
Takeuchi T, Maeta K, Ding X, Oe Y, Takeda A, Inoue M, Nagano S, Fujihara T, Matsuda S, Ishigaki S, Sahashi K, Minakawa EN, Mochizuki H, Neya M, Sobue G, Nagai Y
Molecular therapy. Nucleic acids 2023 Mar 14;31:353-366
Molecular therapy. Nucleic acids 2023 Mar 14;31:353-366
Regulation of RNG105/caprin1 dynamics by pathogenic cytoplasmic FUS and TDP-43 in neuronal RNA granules modulates synaptic loss.
Horio T, Ishikura Y, Ohashi R, Shiina N
Heliyon 2023 Jun;9(6):e17065
Heliyon 2023 Jun;9(6):e17065
Pathologically mislocalised TDP-43 in upper motor neurons causes a die-forward spread of ALS-like pathogenic changes throughout the mouse corticomotor system.
Reale LA, Dyer MS, Perry SE, Young KM, Dickson TC, Woodhouse A, Blizzard CA
Progress in neurobiology 2023 Jul;226:102449
Progress in neurobiology 2023 Jul;226:102449
Atypical TDP-43 protein expression in an ALS pedigree carrying a p.Y374X truncation mutation in TARDBP.
Cooper-Knock J, Julian TH, Feneberg E, Highley JR, Sidra M, Turner MR, Talbot K, Ansorge O, Allen SP, Moll T, Shelkovnikova T, Castelli L, Hautbergue GM, Hewitt C, Kirby J, Wharton SB, Mead RJ, Shaw PJ
Brain pathology (Zurich, Switzerland) 2023 Jan;33(1):e13104
Brain pathology (Zurich, Switzerland) 2023 Jan;33(1):e13104
Pathological Comparison of TDP-43 Between Motor Neurons and Interneurons Expressed by a Tetracycline Repressor System on the Mouse Artificial Chromosome.
Togai S, Hamamichi S, Kazuki Y, Hiratsuka M
Yonago acta medica 2023 Feb;66(1):24-35
Yonago acta medica 2023 Feb;66(1):24-35
Distinctive chaperonopathy in skeletal muscle associated with the dominant variant in DNAJB4.
Inoue M, Noguchi S, Inoue YU, Iida A, Ogawa M, Bengoechea R, Pittman SK, Hayashi S, Watanabe K, Hosoi Y, Sano T, Takao M, Oya Y, Takahashi Y, Miyajima H, Weihl CC, Inoue T, Nishino I
Acta neuropathologica 2023 Feb;145(2):235-255
Acta neuropathologica 2023 Feb;145(2):235-255
NEMO reshapes the α-Synuclein aggregate interface and acts as an autophagy adapter by co-condensation with p62.
Furthmann N, Bader V, Angersbach L, Blusch A, Goel S, Sánchez-Vicente A, Krause LJ, Chaban SA, Grover P, Trinkaus VA, van Well EM, Jaugstetter M, Tschulik K, Damgaard RB, Saft C, Ellrichmann G, Gold R, Koch A, Englert B, Westenberger A, Klein C, Jungbluth L, Sachse C, Behrends C, Glatzel M, Hartl FU, Nakamura K, Christine CW, Huang EJ, Tatzelt J, Winklhofer KF
Nature communications 2023 Dec 19;14(1):8368
Nature communications 2023 Dec 19;14(1):8368
Targeting RACK1 to alleviate TDP-43 and FUS proteinopathy-mediated suppression of protein translation and neurodegeneration.
Zhao B, Cowan CM, Coutts JA, Christy DD, Saraph A, Hsueh SCC, Plotkin SS, Mackenzie IR, Kaplan JM, Cashman NR
Acta neuropathologica communications 2023 Dec 18;11(1):200
Acta neuropathologica communications 2023 Dec 18;11(1):200
TDP-43 pathology and functional deficits in wild-type and ALS/FTD mutant cyclin F mouse models.
van Hummel A, Sabale M, Przybyla M, van der Hoven J, Chan G, Feiten AF, Chung RS, Ittner LM, Ke YD
Neuropathology and applied neurobiology 2023 Apr;49(2):e12902
Neuropathology and applied neurobiology 2023 Apr;49(2):e12902
Astrocytic TDP-43 dysregulation impairs memory by modulating antiviral pathways and interferon-inducible chemokines.
Licht-Murava A, Meadows SM, Palaguachi F, Song SC, Jackvony S, Bram Y, Zhou C, Schwartz RE, Froemke RC, Orr AL, Orr AG
Science advances 2023 Apr 21;9(16):eade1282
Science advances 2023 Apr 21;9(16):eade1282
Extremely Early-Onset Frontotemporal Dementia: A Case Report and Literature Review.
Chu M, Liu L, Nan H, Jiang D, Wang Y, Rosa-Neto P, Piao Y, Wu L
Journal of Alzheimer's disease : JAD 2022;90(3):1139-1151
Journal of Alzheimer's disease : JAD 2022;90(3):1139-1151
Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43.
Jiang YX, Cao Q, Sawaya MR, Abskharon R, Ge P, DeTure M, Dickson DW, Fu JY, Ogorzalek Loo RR, Loo JA, Eisenberg DS
Nature 2022 May;605(7909):304-309
Nature 2022 May;605(7909):304-309
Estrogen Enhances Dendrite Spine Function and Recovers Deficits in Neuroplasticity in the prpTDP-43(A315T) Mouse Model of Amyotrophic Lateral Sclerosis.
Handley EE, Reale LA, Chuckowree JA, Dyer MS, Barnett GL, Clark CM, Bennett W, Dickson TC, Blizzard CA
Molecular neurobiology 2022 May;59(5):2962-2976
Molecular neurobiology 2022 May;59(5):2962-2976
A single-cell atlas of human and mouse white adipose tissue.
Emont MP, Jacobs C, Essene AL, Pant D, Tenen D, Colleluori G, Di Vincenzo A, Jørgensen AM, Dashti H, Stefek A, McGonagle E, Strobel S, Laber S, Agrawal S, Westcott GP, Kar A, Veregge ML, Gulko A, Srinivasan H, Kramer Z, De Filippis E, Merkel E, Ducie J, Boyd CG, Gourash W, Courcoulas A, Lin SJ, Lee BT, Morris D, Tobias A, Khera AV, Claussnitzer M, Pers TH, Giordano A, Ashenberg O, Regev A, Tsai LT, Rosen ED
Nature 2022 Mar;603(7903):926-933
Nature 2022 Mar;603(7903):926-933
Frontotemporal Lobar Degeneration Case with an N-Terminal TUBA4A Mutation Exhibits Reduced TUBA4A Levels in the Brain and TDP-43 Pathology.
Van Schoor E, Vandenbulcke M, Bercier V, Vandenberghe R, van der Zee J, Van Broeckhoven C, Otto M, Hanseeuw B, Van Damme P, Van Den Bosch L, Thal DR
Biomolecules 2022 Mar 12;12(3)
Biomolecules 2022 Mar 12;12(3)
A quantitative biology approach correlates neuronal toxicity with the largest inclusions of TDP-43.
Cascella R, Bigi A, Riffert DG, Gagliani MC, Ermini E, Moretti M, Cortese K, Cecchi C, Chiti F
Science advances 2022 Jul 29;8(30):eabm6376
Science advances 2022 Jul 29;8(30):eabm6376
TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration.
Xie M, Liu YU, Zhao S, Zhang L, Bosco DB, Pang YP, Zhong J, Sheth U, Martens YA, Zhao N, Liu CC, Zhuang Y, Wang L, Dickson DW, Mattson MP, Bu G, Wu LJ
Nature neuroscience 2022 Jan;25(1):26-38
Nature neuroscience 2022 Jan;25(1):26-38
miR-23a suppression accelerates functional decline in the rNLS8 mouse model of TDP-43 proteinopathy.
Tsitkanou S, Della Gatta PA, Abbott G, Wallace MA, Lindsay A, Gerlinger-Romero F, Walker AK, Foletta VC, Russell AP
Neurobiology of disease 2022 Jan;162:105559
Neurobiology of disease 2022 Jan;162:105559
Detection of Pathological Markers of Neurodegenerative Diseases following Microfluidic Direct Conversion of Patient Fibroblasts into Neurons.
Mollinari C, De Dominicis C, Lupacchini L, Sansone L, Caprini D, Casciola CM, Wang Y, Zhao J, Fini M, Russo M, Garaci E, Merlo D
International journal of molecular sciences 2022 Feb 15;23(4)
International journal of molecular sciences 2022 Feb 15;23(4)
Pathological changes induced by Alzheimer's brain inoculation in amyloid-beta plaque-bearing mice.
Lam S, Hérard AS, Boluda S, Petit F, Eddarkaoui S, Cambon K, Brainbank Neuro-CEB Neuropathology Network, Picq JL, Buée L, Duyckaerts C, Haïk S, Dhenain M
Acta neuropathologica communications 2022 Aug 16;10(1):112
Acta neuropathologica communications 2022 Aug 16;10(1):112
Disease-linked TDP-43 hyperphosphorylation suppresses TDP-43 condensation and aggregation.
Gruijs da Silva LA, Simonetti F, Hutten S, Riemenschneider H, Sternburg EL, Pietrek LM, Gebel J, Dötsch V, Edbauer D, Hummer G, Stelzl LS, Dormann D
The EMBO journal 2022 Apr 19;41(8):e108443
The EMBO journal 2022 Apr 19;41(8):e108443
Hsp90 and its co-chaperone Sti1 control TDP-43 misfolding and toxicity.
Lin LT, Razzaq A, Di Gregorio SE, Hong S, Charles B, Lopes MH, Beraldo F, Prado VF, Prado MAM, Duennwald ML
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2021 May;35(5):e21594
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2021 May;35(5):e21594
CdSe Quantum Dots in Human Models Derived from ALS Patients: Characterization, Nuclear Penetration Studies and Multiplexing.
Tosat-Bitrián C, Avis-Bodas A, Porras G, Borrego-Hernández D, García-Redondo A, Martín-Requero A, Palomo V
Nanomaterials (Basel, Switzerland) 2021 Mar 9;11(3)
Nanomaterials (Basel, Switzerland) 2021 Mar 9;11(3)
TDP-43 and PINK1 mediate CHCHD10(S59L) mutation-induced defects in Drosophila and in vitro.
Baek M, Choe YJ, Bannwarth S, Kim J, Maitra S, Dorn GW 2nd, Taylor JP, Paquis-Flucklinger V, Kim NC
Nature communications 2021 Mar 26;12(1):1924
Nature communications 2021 Mar 26;12(1):1924
Cytoplasmic Human TDP-43 Mislocalization Induces Widespread Dendritic Spine Loss in Mouse Upper Motor Neurons.
Dyer MS, Woodhouse A, Blizzard CA
Brain sciences 2021 Jun 30;11(7)
Brain sciences 2021 Jun 30;11(7)
Increasing Brain Permeability of PHA-767491, a Cell Division Cycle 7 Kinase Inhibitor, with Biodegradable Polymeric Nanoparticles.
Rojas-Prats E, Tosat-Bitrián C, Martínez-González L, Nozal V, Pérez DI, Martínez A
Pharmaceutics 2021 Jan 28;13(2)
Pharmaceutics 2021 Jan 28;13(2)
Spreading of TDP-43 pathology via pyramidal tract induces ALS-like phenotypes in TDP-43 transgenic mice.
Ding X, Xiang Z, Qin C, Chen Y, Tian H, Meng L, Xia D, Liu H, Song J, Fu J, Ma M, Wang X
Acta neuropathologica communications 2021 Jan 18;9(1):15
Acta neuropathologica communications 2021 Jan 18;9(1):15
Generation and analysis of innovative genomically humanized knockin SOD1, TARDBP (TDP-43), and FUS mouse models.
Devoy A, Price G, De Giorgio F, Bunton-Stasyshyn R, Thompson D, Gasco S, Allan A, Codner GF, Nair RR, Tibbit C, McLeod R, Ali Z, Noda J, Marrero-Gagliardi A, Brito-Armas JM, Williams C, Öztürk MM, Simon M, O'Neill E, Bryce-Smith S, Harrison J, Atkins G, Corrochano S, Stewart M, Gilthorpe JD, Teboul L, Acevedo-Arozena A, Fisher EMC, Cunningham TJ
iScience 2021 Dec 17;24(12):103463
iScience 2021 Dec 17;24(12):103463
Stress Granule Assembly Can Facilitate but Is Not Required for TDP-43 Cytoplasmic Aggregation.
Fernandes N, Nero L, Lyons SM, Ivanov P, Mittelmeier TM, Bolger TA, Buchan JR
Biomolecules 2020 Sep 25;10(10)
Biomolecules 2020 Sep 25;10(10)
ALS skin fibroblasts reveal oxidative stress and ERK1/2-mediated cytoplasmic localization of TDP-43.
Romano N, Catalani A, Lattante S, Belardo A, Proietti S, Bertini L, Silvestri F, Catalani E, Cervia D, Zolla L, Sabatelli M, Welshhans K, Ceci M
Cellular signalling 2020 Jun;70:109591
Cellular signalling 2020 Jun;70:109591
RBM45 associates with nuclear stress bodies and forms nuclear inclusions during chronic cellular stress and in neurodegenerative diseases.
Collins M, Li Y, Bowser R
Acta neuropathologica communications 2020 Jun 26;8(1):91
Acta neuropathologica communications 2020 Jun 26;8(1):91
Homozygous GRN mutations: new phenotypes and new insights into pathological and molecular mechanisms.
Huin V, Barbier M, Bottani A, Lobrinus JA, Clot F, Lamari F, Chat L, Rucheton B, Fluchère F, Auvin S, Myers P, Gelot A, Camuzat A, Caillaud C, Jornéa L, Forlani S, Saracino D, Duyckaerts C, Brice A, Durr A, Le Ber I
Brain : a journal of neurology 2020 Jan 1;143(1):303-319
Brain : a journal of neurology 2020 Jan 1;143(1):303-319
Neurodegeneration and Motor Deficits in the Absence of Astrogliosis upon Transgenic Mutant TDP-43 Expression in Mature Mice.
Chan G, van Hummel A, van der Hoven J, Ittner LM, Ke YD
The American journal of pathology 2020 Aug;190(8):1713-1722
The American journal of pathology 2020 Aug;190(8):1713-1722
Leukocyte Derived Microvesicles as Disease Progression Biomarkers in Slow Progressing Amyotrophic Lateral Sclerosis Patients.
Sproviero D, La Salvia S, Colombo F, Zucca S, Pansarasa O, Diamanti L, Costa A, Lova L, Giannini M, Gagliardi S, Lauranzano E, Matteoli M, Ceroni M, Malaspina A, Cereda C
Frontiers in neuroscience 2019;13:344
Frontiers in neuroscience 2019;13:344
Multiple System Atrophy With Predominant Striatonigral Degeneration and TAR DNA-Binding Protein of 43 kDa Pathology: An Unusual Variant of Multiple System Atrophy.
Nwabuobi L, Tomishon D, Shneider NA, Fahn S, Vonsattel JP, Cortes E
Movement disorders clinical practice 2019 Nov;6(8):661-666
Movement disorders clinical practice 2019 Nov;6(8):661-666
Amyotrophic lateral sclerosis mutant TDP-43 may cause synaptic dysfunction through altered dendritic spine function.
Jiang T, Handley E, Brizuela M, Dawkins E, Lewis KEA, Clark RM, Dickson TC, Blizzard CA
Disease models & mechanisms 2019 May 17;12(5)
Disease models & mechanisms 2019 May 17;12(5)
Phosphorylated and aggregated TDP-43 with seeding properties are induced upon mutant Huntingtin (mHtt) polyglutamine expression in human cellular models.
Coudert L, Nonaka T, Bernard E, Hasegawa M, Schaeffer L, Leblanc P
Cellular and molecular life sciences : CMLS 2019 Jul;76(13):2615-2632
Cellular and molecular life sciences : CMLS 2019 Jul;76(13):2615-2632
TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates.
Laferrière F, Maniecka Z, Pérez-Berlanga M, Hruska-Plochan M, Gilhespy L, Hock EM, Wagner U, Afroz T, Boersema PJ, Barmettler G, Foti SC, Asi YT, Isaacs AM, Al-Amoudi A, Lewis A, Stahlberg H, Ravits J, De Giorgi F, Ichas F, Bezard E, Picotti P, Lashley T, Polymenidou M
Nature neuroscience 2019 Jan;22(1):65-77
Nature neuroscience 2019 Jan;22(1):65-77
Single-copy expression of an amyotrophic lateral sclerosis-linked TDP-43 mutation (M337V) in BAC transgenic mice leads to altered stress granule dynamics and progressive motor dysfunction.
Gordon D, Dafinca R, Scaber J, Alegre-Abarrategui J, Farrimond L, Scott C, Biggs D, Kent L, Oliver PL, Davies B, Ansorge O, Wade-Martins R, Talbot K
Neurobiology of disease 2019 Jan;121:148-162
Neurobiology of disease 2019 Jan;121:148-162
The role of lysosomes and autophagosomes in frontotemporal lobar degeneration.
Bain HDC, Davidson YS, Robinson AC, Ryan S, Rollinson S, Richardson A, Jones M, Snowden JS, Pickering-Brown S, Mann DMA
Neuropathology and applied neurobiology 2019 Apr;45(3):244-261
Neuropathology and applied neurobiology 2019 Apr;45(3):244-261
mTh1 driven expression of hTDP-43 results in typical ALS/FTLD neuropathological symptoms.
Scherz B, Rabl R, Flunkert S, Rohler S, Neddens J, Taub N, Temmel M, Panzenboeck U, Niederkofler V, Zimmermann R, Hutter-Paier B
PloS one 2018;13(5):e0197674
PloS one 2018;13(5):e0197674
Pathological Proteins Are Transported by Extracellular Vesicles of Sporadic Amyotrophic Lateral Sclerosis Patients.
Sproviero D, La Salvia S, Giannini M, Crippa V, Gagliardi S, Bernuzzi S, Diamanti L, Ceroni M, Pansarasa O, Poletti A, Cereda C
Frontiers in neuroscience 2018;12:487
Frontiers in neuroscience 2018;12:487
A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing.
Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Ramirez Montero D, Ryan VH, Rohatgi R, Shewmaker F, Naik MT, Mittag T, Ayala YM, Fawzi NL
The EMBO journal 2018 Mar 1;37(5)
The EMBO journal 2018 Mar 1;37(5)
Frontotemporal dementia with trans-activation response DNA-binding protein 43 presenting with catatonic syndrome.
Watanabe R, Kawakami I, Onaya M, Higashi S, Arai N, Akiyama H, Hasegawa M, Arai T
Neuropathology : official journal of the Japanese Society of Neuropathology 2018 Jun;38(3):281-287
Neuropathology : official journal of the Japanese Society of Neuropathology 2018 Jun;38(3):281-287
Unexpected similarities between C9ORF72 and sporadic forms of ALS/FTD suggest a common disease mechanism.
Conlon EG, Fagegaltier D, Agius P, Davis-Porada J, Gregory J, Hubbard I, Kang K, Kim D, New York Genome Center ALS Consortium, Phatnani H, Shneider NA, Manley JL
eLife 2018 Jul 13;7
eLife 2018 Jul 13;7
RNA and Protein Interactors with TDP-43 in Human Spinal-Cord Lysates in Amyotrophic Lateral Sclerosis.
Volkening K, Keller BA, Leystra-Lantz C, Strong MJ
Journal of proteome research 2018 Apr 6;17(4):1712-1729
Journal of proteome research 2018 Apr 6;17(4):1712-1729
Split GFP technologies to structurally characterize and quantify functional biomolecular interactions of FTD-related proteins.
Foglieni C, Papin S, Salvadè A, Afroz T, Pinton S, Pedrioli G, Ulrich G, Polymenidou M, Paganetti P
Scientific reports 2017 Oct 25;7(1):14013
Scientific reports 2017 Oct 25;7(1):14013
Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes.
Tanaka Y, Suzuki G, Matsuwaki T, Hosokawa M, Serrano G, Beach TG, Yamanouchi K, Hasegawa M, Nishihara M
Human molecular genetics 2017 Mar 1;26(5):969-988
Human molecular genetics 2017 Mar 1;26(5):969-988
Synapse Dysfunction of Layer V Pyramidal Neurons Precedes Neurodegeneration in a Mouse Model of TDP-43 Proteinopathies.
Handley EE, Pitman KA, Dawkins E, Young KM, Clark RM, Jiang TC, Turner BJ, Dickson TC, Blizzard CA
Cerebral cortex (New York, N.Y. : 1991) 2017 Jul 1;27(7):3630-3647
Cerebral cortex (New York, N.Y. : 1991) 2017 Jul 1;27(7):3630-3647
ALS/FTLD: experimental models and reality.
Tan RH, Ke YD, Ittner LM, Halliday GM
Acta neuropathologica 2017 Feb;133(2):177-196
Acta neuropathologica 2017 Feb;133(2):177-196
Endocytosis regulates TDP-43 toxicity and turnover.
Liu G, Coyne AN, Pei F, Vaughan S, Chaung M, Zarnescu DC, Buchan JR
Nature communications 2017 Dec 12;8(1):2092
Nature communications 2017 Dec 12;8(1):2092
Early Cognitive/Social Deficits and Late Motor Phenotype in Conditional Wild-Type TDP-43 Transgenic Mice.
Alfieri JA, Silva PR, Igaz LM
Frontiers in aging neuroscience 2016;8:310
Frontiers in aging neuroscience 2016;8:310
Mislocated FUS is sufficient for gain-of-toxic-function amyotrophic lateral sclerosis phenotypes in mice.
Shiihashi G, Ito D, Yagi T, Nihei Y, Ebine T, Suzuki N
Brain : a journal of neurology 2016 Sep;139(Pt 9):2380-94
Brain : a journal of neurology 2016 Sep;139(Pt 9):2380-94
Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains.
Kametani F, Obi T, Shishido T, Akatsu H, Murayama S, Saito Y, Yoshida M, Hasegawa M
Scientific reports 2016 Mar 16;6:23281
Scientific reports 2016 Mar 16;6:23281
Increased cytoplasmic TARDBP mRNA in affected spinal motor neurons in ALS caused by abnormal autoregulation of TDP-43.
Koyama A, Sugai A, Kato T, Ishihara T, Shiga A, Toyoshima Y, Koyama M, Konno T, Hirokawa S, Yokoseki A, Nishizawa M, Kakita A, Takahashi H, Onodera O
Nucleic acids research 2016 Jul 8;44(12):5820-36
Nucleic acids research 2016 Jul 8;44(12):5820-36
In vitro prion-like behaviour of TDP-43 in ALS.
Smethurst P, Newcombe J, Troakes C, Simone R, Chen YR, Patani R, Sidle K
Neurobiology of disease 2016 Dec;96:236-247
Neurobiology of disease 2016 Dec;96:236-247
Nuclear bodies reorganize during myogenesis in vitro and are differentially disrupted by expression of FSHD-associated DUX4.
Homma S, Beermann ML, Yu B, Boyce FM, Miller JB
Skeletal muscle 2016 Dec 1;6(1):42
Skeletal muscle 2016 Dec 1;6(1):42
The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity.
Wang W, Wang L, Lu J, Siedlak SL, Fujioka H, Liang J, Jiang S, Ma X, Jiang Z, da Rocha EL, Sheng M, Choi H, Lerou PH, Li H, Wang X
Nature medicine 2016 Aug;22(8):869-78
Nature medicine 2016 Aug;22(8):869-78
Templated Aggregation of TAR DNA-binding Protein of 43 kDa (TDP-43) by Seeding with TDP-43 Peptide Fibrils.
Shimonaka S, Nonaka T, Suzuki G, Hisanaga S, Hasegawa M
The Journal of biological chemistry 2016 Apr 22;291(17):8896-907
The Journal of biological chemistry 2016 Apr 22;291(17):8896-907
Gain-of-function profilin 1 mutations linked to familial amyotrophic lateral sclerosis cause seed-dependent intracellular TDP-43 aggregation.
Tanaka Y, Nonaka T, Suzuki G, Kametani F, Hasegawa M
Human molecular genetics 2016 Apr 1;25(7):1420-33
Human molecular genetics 2016 Apr 1;25(7):1420-33
Short-term suppression of A315T mutant human TDP-43 expression improves functional deficits in a novel inducible transgenic mouse model of FTLD-TDP and ALS.
Ke YD, van Hummel A, Stevens CH, Gladbach A, Ippati S, Bi M, Lee WS, Krüger S, van der Hoven J, Volkerling A, Bongers A, Halliday G, Haass NK, Kiernan M, Delerue F, Ittner LM
Acta neuropathologica 2015 Nov;130(5):661-78
Acta neuropathologica 2015 Nov;130(5):661-78
TARDBP pathogenic mutations increase cytoplasmic translocation of TDP-43 and cause reduction of endoplasmic reticulum Ca²⁺ signaling in motor neurons.
Mutihac R, Alegre-Abarrategui J, Gordon D, Farrimond L, Yamasaki-Mann M, Talbot K, Wade-Martins R
Neurobiology of disease 2015 Mar;75:64-77
Neurobiology of disease 2015 Mar;75:64-77
Evidence of a link between ubiquilin 2 and optineurin in amyotrophic lateral sclerosis.
Osaka M, Ito D, Yagi T, Nihei Y, Suzuki N
Human molecular genetics 2015 Mar 15;24(6):1617-29
Human molecular genetics 2015 Mar 15;24(6):1617-29
RBM45 Modulates the Antioxidant Response in Amyotrophic Lateral Sclerosis through Interactions with KEAP1.
Bakkar N, Kousari A, Kovalik T, Li Y, Bowser R
Molecular and cellular biology 2015 Jul;35(14):2385-99
Molecular and cellular biology 2015 Jul;35(14):2385-99
An acetylation switch controls TDP-43 function and aggregation propensity.
Cohen TJ, Hwang AW, Restrepo CR, Yuan CX, Trojanowski JQ, Lee VM
Nature communications 2015 Jan 5;6:5845
Nature communications 2015 Jan 5;6:5845
Expression of FSHD-related DUX4-FL alters proteostasis and induces TDP-43 aggregation.
Homma S, Beermann ML, Boyce FM, Miller JB
Annals of clinical and translational neurology 2015 Feb;2(2):151-66
Annals of clinical and translational neurology 2015 Feb;2(2):151-66
Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase.
Henriques A, Croixmarie V, Priestman DA, Rosenbohm A, Dirrig-Grosch S, D'Ambra E, Huebecker M, Hussain G, Boursier-Neyret C, Echaniz-Laguna A, Ludolph AC, Platt FM, Walther B, Spedding M, Loeffler JP, Gonzalez De Aguilar JL
Human molecular genetics 2015 Dec 20;24(25):7390-405
Human molecular genetics 2015 Dec 20;24(25):7390-405
Clinicopathological features of the first Asian family having vocal cord and pharyngeal weakness with distal myopathy due to a MATR3 mutation.
Yamashita S, Mori A, Nishida Y, Kurisaki R, Tawara N, Nishikami T, Misumi Y, Ueyama H, Imamura S, Higuchi Y, Hashiguchi A, Higuchi I, Morishita S, Yoshimura J, Uchino M, Takashima H, Tsuji S, Ando Y
Neuropathology and applied neurobiology 2015 Apr;41(3):391-8
Neuropathology and applied neurobiology 2015 Apr;41(3):391-8
TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies.
Goossens J, Vanmechelen E, Trojanowski JQ, Lee VM, Van Broeckhoven C, van der Zee J, Engelborghs S
Acta neuropathologica communications 2015 Apr 1;3:15
Acta neuropathologica communications 2015 Apr 1;3:15
Reversible behavioral phenotypes in a conditional mouse model of TDP-43 proteinopathies.
Alfieri JA, Pino NS, Igaz LM
The Journal of neuroscience : the official journal of the Society for Neuroscience 2014 Nov 12;34(46):15244-59
The Journal of neuroscience : the official journal of the Society for Neuroscience 2014 Nov 12;34(46):15244-59
Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation.
Suárez-Calvet M, Dols-Icardo O, Lladó A, Sánchez-Valle R, Hernández I, Amer G, Antón-Aguirre S, Alcolea D, Fortea J, Ferrer I, van der Zee J, Dillen L, Van Broeckhoven C, Molinuevo JL, Blesa R, Clarimón J, Lleó A
Journal of neurology, neurosurgery, and psychiatry 2014 Jun;85(6):684-91
Journal of neurology, neurosurgery, and psychiatry 2014 Jun;85(6):684-91
Prevention of intestinal obstruction reveals progressive neurodegeneration in mutant TDP-43 (A315T) mice.
Herdewyn S, Cirillo C, Van Den Bosch L, Robberecht W, Vanden Berghe P, Van Damme P
Molecular neurodegeneration 2014 Jun 17;9:24
Molecular neurodegeneration 2014 Jun 17;9:24
An autopsy case of sporadic amyotrophic lateral sclerosis associated with the I113T SOD1 mutation.
Nakamura S, Wate R, Kaneko S, Ito H, Oki M, Tsuge A, Nagashima M, Asayama S, Fujita K, Nakamura M, Maruyama H, Kawakami H, Kusaka H
Neuropathology : official journal of the Japanese Society of Neuropathology 2014 Feb;34(1):58-63
Neuropathology : official journal of the Japanese Society of Neuropathology 2014 Feb;34(1):58-63
Distinct pathways leading to TDP-43-induced cellular dysfunctions.
Yamashita M, Nonaka T, Hirai S, Miwa A, Okado H, Arai T, Hosokawa M, Akiyama H, Hasegawa M
Human molecular genetics 2014 Aug 15;23(16):4345-56
Human molecular genetics 2014 Aug 15;23(16):4345-56
TDP-43, an ALS linked protein, regulates fat deposition and glucose homeostasis.
Stallings NR, Puttaparthi K, Dowling KJ, Luther CM, Burns DK, Davis K, Elliott JL
PloS one 2013;8(8):e71793
PloS one 2013;8(8):e71793
Cytosolic 5'-nucleotidase 1A autoimmunity in sporadic inclusion body myositis.
Larman HB, Salajegheh M, Nazareno R, Lam T, Sauld J, Steen H, Kong SW, Pinkus JL, Amato AA, Elledge SJ, Greenberg SA
Annals of neurology 2013 Mar;73(3):408-18
Annals of neurology 2013 Mar;73(3):408-18
Ubiquilin-2 (UBQLN2) binds with high affinity to the C-terminal region of TDP-43 and modulates TDP-43 levels in H4 cells: characterization of inhibition by nucleic acids and 4-aminoquinolines.
Cassel JA, Reitz AB
Biochimica et biophysica acta 2013 Jun;1834(6):964-71
Biochimica et biophysica acta 2013 Jun;1834(6):964-71
Prion-like properties of pathological TDP-43 aggregates from diseased brains.
Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, Yoshida M, Murayama S, Mann DM, Akiyama H, Hasegawa M
Cell reports 2013 Jul 11;4(1):124-34
Cell reports 2013 Jul 11;4(1):124-34
A unique mouse model for investigating the properties of amyotrophic lateral sclerosis-associated protein TDP-43, by in utero electroporation.
Akamatsu M, Takuma H, Yamashita T, Okada T, Keino-Masu K, Ishii K, Kwak S, Masu M, Tamaoka A
Neuroscience research 2013 Dec;77(4):234-41
Neuroscience research 2013 Dec;77(4):234-41
The dual functions of the extreme N-terminus of TDP-43 in regulating its biological activity and inclusion formation.
Zhang YJ, Caulfield T, Xu YF, Gendron TF, Hubbard J, Stetler C, Sasaguri H, Whitelaw EC, Cai S, Lee WC, Petrucelli L
Human molecular genetics 2013 Aug 1;22(15):3112-22
Human molecular genetics 2013 Aug 1;22(15):3112-22
Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination.
Dammer EB, Fallini C, Gozal YM, Duong DM, Rossoll W, Xu P, Lah JJ, Levey AI, Peng J, Bassell GJ, Seyfried NT
PloS one 2012;7(6):e38658
PloS one 2012;7(6):e38658
Molecular analysis and biochemical classification of TDP-43 proteinopathy.
Tsuji H, Arai T, Kametani F, Nonaka T, Yamashita M, Suzukake M, Hosokawa M, Yoshida M, Hatsuta H, Takao M, Saito Y, Murayama S, Akiyama H, Hasegawa M, Mann DM, Tamaoka A
Brain : a journal of neurology 2012 Nov;135(Pt 11):3380-91
Brain : a journal of neurology 2012 Nov;135(Pt 11):3380-91
Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking.
Cohen TJ, Hwang AW, Unger T, Trojanowski JQ, Lee VM
The EMBO journal 2012 Mar 7;31(5):1241-52
The EMBO journal 2012 Mar 7;31(5):1241-52
Epitope mapping of antibodies against TDP-43 and detection of protease-resistant fragments of pathological TDP-43 in amyotrophic lateral sclerosis and frontotemporal lobar degeneration.
Tsuji H, Nonaka T, Yamashita M, Masuda-Suzukake M, Kametani F, Akiyama H, Mann DM, Tamaoka A, Hasegawa M
Biochemical and biophysical research communications 2012 Jan 6;417(1):116-21
Biochemical and biophysical research communications 2012 Jan 6;417(1):116-21
Association of UBQLN1 mutation with Brown-Vialetto-Van Laere syndrome but not typical ALS.
González-Pérez P, Lu Y, Chian RJ, Sapp PC, Tanzi RE, Bertram L, McKenna-Yasek D, Gao FB, Brown RH Jr
Neurobiology of disease 2012 Dec;48(3):391-8
Neurobiology of disease 2012 Dec;48(3):391-8
Inclusion body myositis coexisting with hypertrophic cardiomyopathy: an autopsy study.
Inamori Y, Higuchi I, Inoue T, Sakiyama Y, Hashiguchi A, Higashi K, Shiraishi T, Okubo R, Arimura K, Mitsuyama Y, Takashima H
Neuromuscular disorders : NMD 2012 Aug;22(8):747-54
Neuromuscular disorders : NMD 2012 Aug;22(8):747-54
AMSH is required to degrade ubiquitinated proteins in the central nervous system.
Suzuki S, Tamai K, Watanabe M, Kyuuma M, Ono M, Sugamura K, Tanaka N
Biochemical and biophysical research communications 2011 May 20;408(4):582-8
Biochemical and biophysical research communications 2011 May 20;408(4):582-8
Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice.
Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, Lee VM
The Journal of clinical investigation 2011 Feb;121(2):726-38
The Journal of clinical investigation 2011 Feb;121(2):726-38
Dysfunction of the ubiquitin-proteasome system in the cerebellum of aging Ts65Dn mice.
Necchi D, Lomoio S, Scherini E
Experimental neurology 2011 Dec;232(2):114-8
Experimental neurology 2011 Dec;232(2):114-8
Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia.
Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T
Nature 2011 Aug 21;477(7363):211-5
Nature 2011 Aug 21;477(7363):211-5
FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis.
Deng HX, Zhai H, Bigio EH, Yan J, Fecto F, Ajroud K, Mishra M, Ajroud-Driss S, Heller S, Sufit R, Siddique N, Mugnaini E, Siddique T
Annals of neurology 2010 Jun;67(6):739-48
Annals of neurology 2010 Jun;67(6):739-48
Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43.
Nishimoto Y, Ito D, Yagi T, Nihei Y, Tsunoda Y, Suzuki N
The Journal of biological chemistry 2010 Jan 1;285(1):608-19
The Journal of biological chemistry 2010 Jan 1;285(1):608-19
Caspase-cleaved TAR DNA-binding protein-43 is a major pathological finding in Alzheimer's disease.
Rohn TT
Brain research 2008 Sep 4;1228:189-98
Brain research 2008 Sep 4;1228:189-98
TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
Steinacker P, Hendrich C, Sperfeld AD, Jesse S, von Arnim CA, Lehnert S, Pabst A, Uttner I, Tumani H, Lee VM, Trojanowski JQ, Kretzschmar HA, Ludolph A, Neumann M, Otto M
Archives of neurology 2008 Nov;65(11):1481-7
Archives of neurology 2008 Nov;65(11):1481-7
TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system.
Lee EB, Lee VM, Trojanowski JQ, Neumann M
Acta neuropathologica 2008 Mar;115(3):305-11
Acta neuropathologica 2008 Mar;115(3):305-11
No comments: Submit comment
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image

- Experimental details
- human placenta tissue were subjected to SDS PAGE followed by western blot with 60019-2-Ig(TARDBP antibody) at dilution of 1:300
- Sample type
- tissue
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image
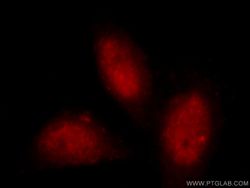
- Experimental details
- Immunofluorescent analysis of MCF-7 cells, using TARDBP antibody 60019-2-lg at 1:25 dilution and Rhodamine-labeled goat anti-mouse IgG (red).
- Sample type
- cell line
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image
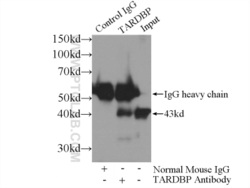
- Experimental details
- IP Result of anti-TDP-43 (IP:60019-2-Ig, 5ug; Detection:60019-2-Ig 1:1000) with K-562 cells lysate 1720ug.
- Sample type
- cell line
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image
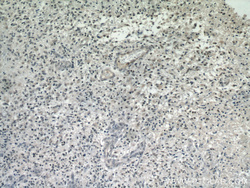
- Experimental details
- Immunohistochemical of paraffin-embedded human gliomas using 60019-2-Ig(TARDBP antibody) at dilution of 1:50 (under 10x lens)
- Sample type
- tissue
- Submitted by
- Proteintech Group (provider)
- Main image
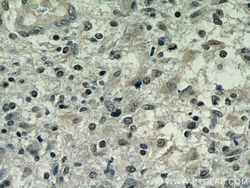
- Experimental details
- Immunohistochemical of paraffin-embedded human gliomas using 60019-2-Ig(TARDBP antibody) at dilution of 1:50 (under 40x lens)
- Sample type
- tissue
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA