MA1-054
antibody from Invitrogen Antibodies
Targeting: ADIPOQ
ACDC, ACRP30, adiponectin, apM1, GBP28
Antibody data
- Antibody Data
- Antigen structure
- References [21]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [2]
- Flow cytometry [1]
- Other assay [13]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA1-054 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Adiponectin Monoclonal Antibody (19F1)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- MA1-054 detects adiponectin from human, rabbit and mouse samples. MA1-054 has been used successfully in Western blot, immunofluorescence, and ELISA procedures. By Western blot this antibody detects ~30 kDa protein representing Adiponectin in 3T3-L1 adipocytes. Immunofluorescence and ELISA of Adiponectin can also be performed using MA1-054. The MA1-054 immunogen is full length, recombinant, human adiponectin.
- Reactivity
- Human, Mouse, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 19F1
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references The autophagy protein Becn1 improves insulin sensitivity by promoting adiponectin secretion via exocyst binding.
LIPE-related lipodystrophic syndrome: clinical features and disease modeling using adipose stem cells.
EPHX1 mutations cause a lipoatrophic diabetes syndrome due to impaired epoxide hydrolysis and increased cellular senescence.
Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery.
Site-Dependent Lineage Preference of Adipose Stem Cells.
Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds.
TAZ Is a Negative Regulator of PPARγ Activity in Adipocytes and TAZ Deletion Improves Insulin Sensitivity and Glucose Tolerance.
Knockdown of Ant2 Reduces Adipocyte Hypoxia And Improves Insulin Resistance in Obesity.
Regulator of Calcineurin 1 helps coordinate whole-body metabolism and thermogenesis.
Prospective validation of a novel renal activity index of lupus nephritis.
Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia.
Effect of 12 Weeks of Periodized Resistance Training Upon Total Plasma Adiponectin Concentration in Healthy Young Men.
St. John's Wort Has Metabolically Favorable Effects on Adipocytes In Vivo.
Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction.
Naringenin inhibits adipogenesis and reduces insulin sensitivity and adiponectin expression in adipocytes.
The transcription factor paired-related homeobox 1 (Prrx1) inhibits adipogenesis by activating transforming growth factor-β (TGFβ) signaling.
Biochemical changes induced by strontium ranelate in differentiating adipocytes.
Snail, a transcriptional regulator, represses adiponectin expression by directly binding to an E-box motif in the promoter.
LTP induction translocates cortactin at distant synapses in wild-type but not Fmr1 knock-out mice.
HIV protease inhibitors activate the adipocyte renin angiotensin system.
Mechanisms regulating repression of haptoglobin production by peroxisome proliferator-activated receptor-gamma ligands in adipocytes.
Kuramoto K, Kim YJ, Hong JH, He C
Cell reports 2021 May 25;35(8):109184
Cell reports 2021 May 25;35(8):109184
LIPE-related lipodystrophic syndrome: clinical features and disease modeling using adipose stem cells.
Sollier C, Capel E, Aguilhon C, Smirnov V, Auclair M, Douillard C, Ladsous M, Defoort-Dhellemmes S, Gorwood J, Braud L, Motterlini R, Vatier C, Lascols O, Renard E, Vigouroux C, Jéru I
European journal of endocrinology 2021 Jan;184(1):155-168
European journal of endocrinology 2021 Jan;184(1):155-168
EPHX1 mutations cause a lipoatrophic diabetes syndrome due to impaired epoxide hydrolysis and increased cellular senescence.
Gautheron J, Morisseau C, Chung WK, Zammouri J, Auclair M, Baujat G, Capel E, Moulin C, Wang Y, Yang J, Hammock BD, Cerame B, Phan F, Fève B, Vigouroux C, Andreelli F, Jeru I
eLife 2021 Aug 3;10
eLife 2021 Aug 3;10
Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery.
Di Franco S, Bianca P, Sardina DS, Turdo A, Gaggianesi M, Veschi V, Nicotra A, Mangiapane LR, Lo Iacono M, Pillitteri I, van Hooff S, Martorana F, Motta G, Gulotta E, Lentini VL, Martorana E, Fiori ME, Vieni S, Bongiorno MR, Giannone G, Giuffrida D, Memeo L, Colarossi L, Mare M, Vigneri P, Todaro M, De Maria R, Medema JP, Stassi G
Nature communications 2021 Aug 18;12(1):5006
Nature communications 2021 Aug 18;12(1):5006
Site-Dependent Lineage Preference of Adipose Stem Cells.
Wang T, Hill RC, Dzieciatkowska M, Zhu L, Infante AM, Hu G, Hansen KC, Pei M
Frontiers in cell and developmental biology 2020;8:237
Frontiers in cell and developmental biology 2020;8:237
Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds.
Jorgensen AM, Varkey M, Gorkun A, Clouse C, Xu L, Chou Z, Murphy SV, Molnar J, Lee SJ, Yoo JJ, Soker S, Atala A
Tissue engineering. Part A 2020 May;26(9-10):512-526
Tissue engineering. Part A 2020 May;26(9-10):512-526
TAZ Is a Negative Regulator of PPARγ Activity in Adipocytes and TAZ Deletion Improves Insulin Sensitivity and Glucose Tolerance.
El Ouarrat D, Isaac R, Lee YS, Oh DY, Wollam J, Lackey D, Riopel M, Bandyopadhyay G, Seo JB, Sampath-Kumar R, Olefsky JM
Cell metabolism 2020 Jan 7;31(1):162-173.e5
Cell metabolism 2020 Jan 7;31(1):162-173.e5
Knockdown of Ant2 Reduces Adipocyte Hypoxia And Improves Insulin Resistance in Obesity.
Seo JB, Riopel M, Cabrales P, Huh JY, Bandyopadhyay GK, Andreyev AY, Murphy AN, Beeman SC, Smith GI, Klein S, Lee YS, Olefsky JM
Nature metabolism 2019 Jan;1(1):86-97
Nature metabolism 2019 Jan;1(1):86-97
Regulator of Calcineurin 1 helps coordinate whole-body metabolism and thermogenesis.
Rotter D, Peiris H, Grinsfelder DB, Martin AM, Burchfield J, Parra V, Hull C, Morales CR, Jessup CF, Matusica D, Parks BW, Lusis AJ, Nguyen NUN, Oh M, Iyoke I, Jakkampudi T, McMillan DR, Sadek HA, Watt MJ, Gupta RK, Pritchard MA, Keating DJ, Rothermel BA
EMBO reports 2018 Dec;19(12)
EMBO reports 2018 Dec;19(12)
Prospective validation of a novel renal activity index of lupus nephritis.
Gulati G, Bennett MR, Abulaban K, Song H, Zhang X, Ma Q, Brodsky SV, Nadasdy T, Haffner C, Wiley K, Ardoin SP, Devarajan P, Ying J, Rovin BH, Brunner HI
Lupus 2017 Aug;26(9):927-936
Lupus 2017 Aug;26(9):927-936
Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia.
Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, Sulston RJ, Burr AA, Das AK, Simon BR, Mori H, Bree AJ, Schell B, Krishnan V, MacDougald OA
Endocrinology 2016 Feb;157(2):508-21
Endocrinology 2016 Feb;157(2):508-21
Effect of 12 Weeks of Periodized Resistance Training Upon Total Plasma Adiponectin Concentration in Healthy Young Men.
Davis GR, Stephens JM, Nelson AG
Journal of strength and conditioning research 2015 Nov;29(11):3097-104
Journal of strength and conditioning research 2015 Nov;29(11):3097-104
St. John's Wort Has Metabolically Favorable Effects on Adipocytes In Vivo.
Fuller S, Richard AJ, Ribnicky DM, Beyl R, Mynatt R, Stephens JM
Evidence-based complementary and alternative medicine : eCAM 2014;2014:862575
Evidence-based complementary and alternative medicine : eCAM 2014;2014:862575
Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction.
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA
Cell metabolism 2014 Aug 5;20(2):368-375
Cell metabolism 2014 Aug 5;20(2):368-375
Naringenin inhibits adipogenesis and reduces insulin sensitivity and adiponectin expression in adipocytes.
Richard AJ, Amini-Vaughan Z, Ribnicky DM, Stephens JM
Evidence-based complementary and alternative medicine : eCAM 2013;2013:549750
Evidence-based complementary and alternative medicine : eCAM 2013;2013:549750
The transcription factor paired-related homeobox 1 (Prrx1) inhibits adipogenesis by activating transforming growth factor-β (TGFβ) signaling.
Du B, Cawthorn WP, Su A, Doucette CR, Yao Y, Hemati N, Kampert S, McCoin C, Broome DT, Rosen CJ, Yang G, MacDougald OA
The Journal of biological chemistry 2013 Feb 1;288(5):3036-47
The Journal of biological chemistry 2013 Feb 1;288(5):3036-47
Biochemical changes induced by strontium ranelate in differentiating adipocytes.
Vidal C, Gunaratnam K, Tong J, Duque G
Biochimie 2013 Apr;95(4):793-8
Biochimie 2013 Apr;95(4):793-8
Snail, a transcriptional regulator, represses adiponectin expression by directly binding to an E-box motif in the promoter.
Park YM, Lee YH, Kim SH, Lee EY, Kim KS, Williams DR, Lee HC
Metabolism: clinical and experimental 2012 Nov;61(11):1622-32
Metabolism: clinical and experimental 2012 Nov;61(11):1622-32
LTP induction translocates cortactin at distant synapses in wild-type but not Fmr1 knock-out mice.
Seese RR, Babayan AH, Katz AM, Cox CD, Lauterborn JC, Lynch G, Gall CM
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 May 23;32(21):7403-13
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 May 23;32(21):7403-13
HIV protease inhibitors activate the adipocyte renin angiotensin system.
Boccara F, Auclair M, Cohen A, Lefèvre C, Prot M, Bastard JP, Capeau J, Caron-Debarle M
Antiviral therapy 2010;15(3):363-75
Antiviral therapy 2010;15(3):363-75
Mechanisms regulating repression of haptoglobin production by peroxisome proliferator-activated receptor-gamma ligands in adipocytes.
Vernochet C, Davis KE, Scherer PE, Farmer SR
Endocrinology 2010 Feb;151(2):586-94
Endocrinology 2010 Feb;151(2):586-94
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
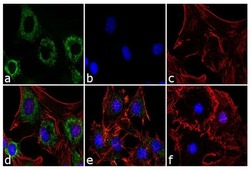
- Experimental details
- Immunofluorescence analysis of Adiponectin was performed using 70% confluent 3T3-L1 cells differentiated with StemPro Adipogenesis Supplement (Product # A10070-01) for 5 days. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Adiponectin (19F1) Mouse Monoclonal Antibody (Product # MA1-054) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) a dilution of 1:2,000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e is untreated cell with less signal. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
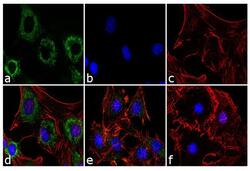
- Experimental details
- Immunofluorescence analysis of Adiponectin was performed using 70% confluent 3T3-L1 cells differentiated with StemPro Adipogenesis Supplement (Product # A10070-01) for 5 days. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Adiponectin (19F1) Mouse Monoclonal Antibody (Product # MA1-054) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A28175) a dilution of 1:2,000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic localization. Panel e is untreated cell with less signal. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
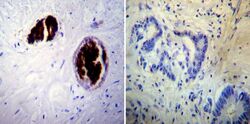
- Experimental details
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human colon carcinoma tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing Adiponectin (Product # MA1-054) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
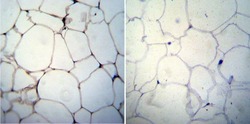
- Experimental details
- Immunohistochemistry was performed on biopsies of deparaffinized Human skin tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing Adiponectin (Product # MA1-054) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
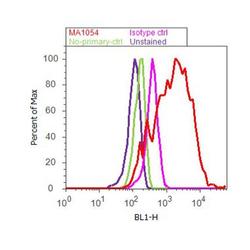
- Experimental details
- Flow cytometry analysis of Adiponectin was performed using 3T3-L1 cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Adiponectin Mouse Monoclonal Antibody (Product # MA1-054, red histogram) or with mouse isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Rabbit Anti-Mouse Secondary Antibody (Product # A11059) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10, 000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
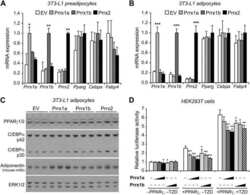
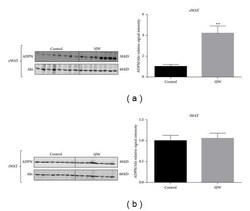
- Figure 3 SJW increases high molecular weight (HMW) adiponectin in epididymal (eWAT) and inguinal (iWAT) fat depots. Proteins from eWAT and iWAT were separated by native PAGE and visualized by western blot analysis. Bar graphs represent relative optical densities of HMW adiponectin, normalized to those in the control group. N = 8/group. Data are presented as means +- SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
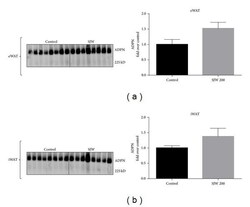
- Figure 2 SJW increases the levels of adiponectin in WAT in a depot specific manner. WAT from the epididymal (a) and inguinal (b) depots was removed and immediately frozen in liquid nitrogen. Tissue extracts were subjected to western blot analysis. There were 13 mice per condition and, of those, 8 mice per condition are shown. Bar graphs represent relative optical densities of adiponectin, normalized to total Akt derived from the same gel. N = 8/group. ** P < 0.01 versus control. Data are presented as means +- SEM.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4 SJW does not affect the serum levels of high molecular weight (a) and total adiponectin (b) in chow-fed C57BL/6J mice. Serum proteins were separated by native PAGE and SDS-PAGE (resp., for HMW and total adiponectin (ADPN) content) and visualized by western blot analysis. Densitometric analysis is shown below each blot. Data are means +- SEM. N = 8/group for HMW ADPN. For total ADPN, N = 6 for controls and N = 8 for SJW.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
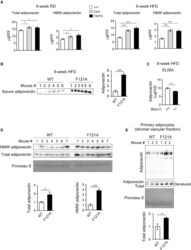
- Experimental details
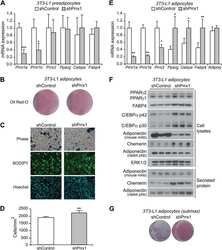
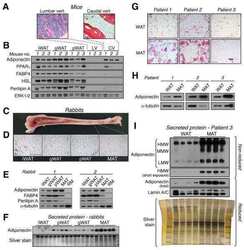
- Figure 2. Becn1 positively regulates adiponectin secretion in vivo and in vitro (A) ELISA of total adiponectin and HMW adiponectin in the serum of WT (+/+) mice and heterozygous (FA/+) or homozygous (FA/FA) Becn1 F121A KI mice fed with RD (regular diet) or HFD for 8 weeks. Total adiponectin, n = 8-17; HMW adiponectin, n = 7-11. One-way ANOVA with Dunnett's test. (B) WB analysis of adiponectin in the serum of WT and Becn1 F121A mice fed with HFD for 8 weeks. n = 6. (C) ELISA of serum adiponectin in WT littermates (+/+) and Becn1 +/- KO (+/-) mice fed with HFD for 8 weeks. +/+, n = 12; +/-, n = 10. (D) Total and HMW adiponectin are increased in Becn1 F121A mice. WB analysis of HMW and total adiponectin in the serum of WT and Becn1 F121A mice using a mouse anti-adiponectin antibody (MA1-054; Thermo Fisher Scientific). Total protein loading is shown by Ponceau S staining. n = 7. (E) Becn1 F121A -induced adiponectin secretion is cell autonomous. Primary adipocytes (adipose-derived stromal vascular fraction [SVF] cells) isolated from Becn1 F121A mice secrete more adiponectin into the conditioned medium than those from WT mice, quantified by WB analysis after 24-h culture. Total protein loading is shown by Ponceau S staining. n = 3 mice. Data represent mean +- SEM, t test. *p < 0.05; **p < 0.01; ***p < 0.001.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
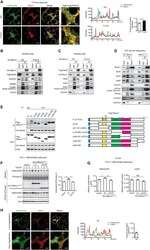
- Experimental details
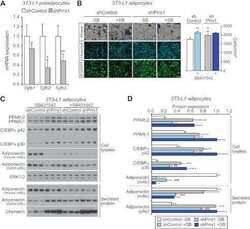
- Figure 5. Becn1 forms a complex with the exocyst, and the Becn1-exocyst interaction is essential to increase secretion of adiponectin, but not leptin, in Becn1 F121A adipocytes (A) Immunofluorescence staining of adiponectin (green) and Becn1 (red) in SVF primary adipocytes isolated from WT or Becn1 F121A mice. The intensity line profile measurements show intensity distribution on dashed arrows in the images above. Pearson correlation coefficient is used to quantify colocalization between adiponectin and Becn1. Low magnification, scale bars: 10 mum; high magnification, scale bars: 5 mum. n = 7 cells. t test. (B) The exocyst component Sec6, but not Exo84 or Exo70, is pulled down by Becn1 F121A more strongly than WT Becn1. CoIP of FLAG-Sec6 by HA-Becn1 in HEK293 cells transfected with Sec6 and WT Becn1 or Becn1 F121A . (C) Becn1 F121A promotes exocyst assembly. CoIP of HA-Becn1, Sec8, and Sec5 by FLAG-Sec6 in HEK293 cells transfected with Sec6 and WT Becn1 or Becn1 F121A . (D) CoIP of endogenous exocyst components (Sec6, Sec5, and Sec8) and autophagy-related PtdIns3K proteins (Atg14, Vps34, and UVRAG) with the endogenous Becn1 protein in primary adipocytes isolated from WT or Becn1 F121A mice. One-way ANOVA with Tukey-Kramer test. (E) CoIP of HA-hSec6 (human Sec6) and indicated full-length (FL) and deletion mutants of FLAG-hBecn1 F123A (human Becn1 F123A , mouse Becn1 F121A equivalent) in HEK293 cells. (F) WB analysis of adiponectin levels in the conditioned medium secreted from
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
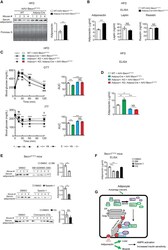
- Experimental details
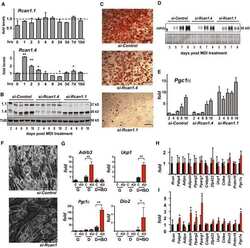
- Figure 7. Adipose-specific Becn1 F121A non-cell-autonomously improves systemic insulin sensitivity via promoting adiponectin secretion (A) WB analysis of serum adiponectin levels in Adipoq-Cre or control (WT) mice injected with AAV2/8-FLEX-Becn1 F121A and then fed with HFD for 12 weeks. n = 4. t test. (B) ELISA of serum levels of adiponectin, resistin, and leptin in Adipoq-Cre or WT mice injected with AAV2/8-FLEX-Becn1 F121A and then fed with HFD for 12 weeks. WT+AAV-Becn1 F121A , n = 11; Adipoq-Cre+AAV-Becn1 F121A , n = 9. t test. (C and D) Reducing adiponectin in adipose-specific Becn1 F121A mice abolishes the insulin-sensitizing effects of adipose Becn1 F121A . GTT and ITT (C) and ELISA of serum adiponectin (D) of WT and adipose-specific Becn1 F121A mice expressing both or one copy of Adiponectin after 8 weeks of HFD feeding. For GTT and ITT: WT+AAV-Becn1 F121A , n = 9; Adipoq-Cre+AAV-Becn1 F121A , n = 9; Adipoq +/- KO+AAV-Becn1 F121A , n = 6; Adipoq +/- KO Adipoq-Cre+AAV-Becn1 F121A , n = 5. For adiponectin ELISA: n = 5-6. One-way ANOVA with Dunnett's test. (E) Inhibiting early (upper), but not late (lower), stages of autophagy reduces adiponectin release to circulation in mice. WB analysis of adiponectin in the serum of HFD-fed Becn1 F121A mice treated with SBI-0206965 (SBI) or Spautin-1 (early-stage inhibitors, inhibiting autophagosome formation) or chloroquine (CQ; late-stage inhibitor, inhibiting lysosomal degradation). n = 4-6. t test. (F) ELISA of serum adiponectin
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
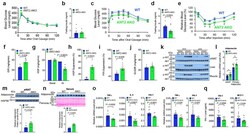
- Experimental details
- Figure 3 ANT2 AKO mice are protected from HFD-induced adipose tissue inflammation, glucose intolerance and insulin resistance. ( a ) GTTs in NCD mice (WT, n=10; ANT2 AKO, n=5). ( b ) Fasting plasma insulin levels in NCD mice (n=11 and 6 mice for WT and ANT2 AKO, respectively). ( c ) GTTs in 12 week HFD mice (n=11 and 8 mice for WT and ANT2 AKO, respectively). a P =0.008, b P =0.043, c P =0.028, according to the two-way ANOVA with post-hoc two-tailed t-tests between the individual groups. ( d ) Fasting plasma insulin levels in HFD mice (n=8 and 11 mice for WT and ANT2 AKO, respectively). ( e ) ITTs in HFD mice for 12 weeks (n=8 and 11 mice for WT and ANT2 AKO, respectively). a P =0.020, b P =0.002, c P =0.030, d P =0.005, e P =0.005 according to the two-way ANOVA with post-hoc two-tailed t-tests between the individual groups. ( f - j ) Systemic and tissue-specific effects of ANT2 AKO was determined by hyperinsulinemic euglycemic clamp studies in 12 week HFD mice (n=4 and 6 mice for WT and ANT2 AKO, respectively). ( f ) GIR. ( g ) HGP. ( h ) HGP suppression. ( i ) Suppression of plasma FFA levels. ( j ) IS-GDR. ( k ) Akt phosphorylation in eWAT, liver, and muscle of WT and ANT2 AKO mice. Representative data from two independent experiments are shown. ( l ) mRNA levels of adiponectin in eWAT and primary adipocytes of HFD WT and ANT2 AKO mice (n=8 mice per group). * P =0.027, xi P =0.018. ( m , n ) Western blot analysis of adiponectin protein levels in eWAT ( m ; n=12 mice per gr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
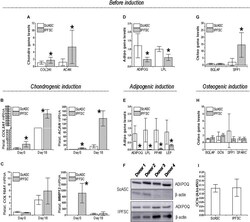
- FIGURE 3 Expression of differentiation genes in ScASCs ( n = 4) and donor-matched IPFSCs ( n = 4) before and after induction. For chondrogenic differentiation, typical markers, COL2A1 and ACAN , were evaluated using qPCR before (A) and after chondrogenic induction (B) . Hypertrophic markers COL10A1 and MMP13 were also measured after chondrogenic induction (C) . For adipogenic differentiation, typical markers, ADIPOQ and LPL , were evaluated using qPCR before (D) and after adipogenic induction (E) . LEP expression was also measured after induction (E) . Adiponectin (ADIPOQ) expression was confirmed using Western blot analysis with beta-actin as an internal control (F) . For osteogenic differentiation, typical markers, BGLAP and SPP1 , were evaluated using qPCR before (G) and after osteogenic induction (H) . Expression of DCN and SPARC were also measured after induction (H) . Osteocalcin (OCN) expression was confirmed using ELISA analysis (I) . Expression of each target gene in IPFSCs is plotted against the corresponding ScASCs, which is set as ""1"", but for those in chondrogenic induction (B/C) , expression in day 0 is plotted as ""1"". Data are shown as bar charts. * indicates a significant difference compared to the corresponding control group ( p < 0.05).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA Immunocytochemistry
Immunocytochemistry