Antibody data
- Antibody Data
- Antigen structure
- References [84]
- Comments [0]
- Validations
- Other assay [38]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-6988-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- ROR gamma (t) Monoclonal Antibody (AFKJS-9), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The AFKJS-9 monoclonal antibody reacts with the mouse, human and rhesus monkey ROR gamma (t) protein. ROR gamma is a member of the retinoic acid-related orphan receptor (ROR) family, which also includes ROR alpha and ROR beta. ROR family proteins are ligand-dependent transcription factors that play roles in multiple physiological processes. ROR gamma is expressed in several tissues including liver, lung, muscle, heart and kidney. Furthermore, it was discovered that alternative transcription results in the expression of an isoform, ROR gamma (t), which is expressed exclusively in cells of the lymphoid compartment, namely CD4+CD8+ "double-positive" thymocytes, Th17 cells of the periphery and lymphoid tissue inducer (LTi) cells of lymphoid organs. The ROR gamma (t) isoform differs from ROR gamma by three unique amino acids at its amino terminus. Therefore, the AFKJS-9 antibody will react with both the ROR gamma and ROR gamma (t) isoforms. Applications Reported: This AFKJS-9 antibody has been reported for use in chromatin immunoprecipitation and western blotting. Applications Tested: This AFKJS-9 antibody has been tested by western blot analysis. This can be used at less than or equal to 5 µg per mL. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human, Mouse
- Host
- Rat
- Isotype
- IgG
- Antibody clone number
- AFKJS-9
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4° C
Submitted references CYP11A1‑derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas.
Iron controls T helper cell pathogenicity by promoting glucose metabolism in autoimmune myopathy.
ILC3s control airway inflammation by limiting T cell responses to allergens and microbes.
AMPK induces regulatory innate lymphoid cells after traumatic brain injury.
Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes.
CD2(+) T-helper 17-like cells differentiated from a CD133(+) subpopulation of non-small cell lung carcinoma cells promote the growth of lung carcinoma.
Abnormal Eating Patterns Cause Circadian Disruption and Promote Alcohol-Associated Colon Carcinogenesis.
Intestinal vitamin D receptor knockout protects from oxazolone-induced colitis.
Ahr-Foxp3-RORγt axis controls gut homing of CD4(+) T cells by regulating GPR15.
Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer.
JQ1, a bromodomain inhibitor, suppresses Th17 effectors by blocking p300-mediated acetylation of RORγt.
TSLP Produced by Aspergillus fumigatus-Stimulated DCs Promotes a Th17 Response Through the JAK/STAT Signaling Pathway in Fungal Keratitis.
BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis.
The colonic macrophage transcription factor RBP-J orchestrates intestinal immunity against bacterial pathogens.
Protein arginine methyltransferase 5 promotes cholesterol biosynthesis-mediated Th17 responses and autoimmunity.
Establishment of Chronic Typhoid Infection in a Mouse Carriage Model Involves a Type 2 Immune Shift and T and B Cell Recruitment to the Gallbladder.
Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity.
BCAP links IL-1R to the PI3K-mTOR pathway and regulates pathogenic Th17 cell differentiation.
Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer.
Inflammation-induced Id2 promotes plasticity in regulatory T cells.
Human CD40 ligand-expressing type 3 innate lymphoid cells induce IL-10-producing immature transitional regulatory B cells.
Trim33 mediates the proinflammatory function of Th17 cells.
Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation.
High dietary salt intake correlates with modulated Th17-Treg cell balance resulting in enhanced bone loss and impaired bone-microarchitecture in male mice.
Fisetin inhibits pristine-induced systemic lupus erythematosus in a murine model through CXCLs regulation.
Epigenetic activation during T helper 17 cell differentiation is mediated by Tripartite motif containing 28.
Characterisation of innate lymphoid cell populations at different sites in mice with defective T cell immunity.
JunB is essential for IL-23-dependent pathogenicity of Th17 cells.
Cutting Edge: Selective Oral ROCK2 Inhibitor Reduces Clinical Scores in Patients with Psoriasis Vulgaris and Normalizes Skin Pathology via Concurrent Regulation of IL-17 and IL-10.
Protein kinase CK2 controls T-cell polarization through dendritic cell activation in response to contact sensitizers.
CD40-signalling abrogates induction of RORγt(+) Treg cells by intestinal CD103(+) DCs and causes fatal colitis.
WASH maintains NKp46(+) ILC3 cells by promoting AHR expression.
High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice.
PRMT5-Selective Inhibitors Suppress Inflammatory T Cell Responses and Experimental Autoimmune Encephalomyelitis.
IL-1β, But Not Programed Death-1 and Programed Death Ligand Pathway, Is Critical for the Human Th17 Response to Mycobacterium tuberculosis.
An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells.
Human mast cells capture, store, and release bioactive, exogenous IL-17A.
Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin α1β2 in Newly Formed Tertiary Lymphoid Structures.
Protein C receptor (PROCR) is a negative regulator of Th17 pathogenicity.
Development of Th17-Associated Interstitial Kidney Inflammation in Lupus-Prone Mice Lacking the Gene Encoding STAT-1.
ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer.
B-cell anergy induces a Th17 shift in a novel B lymphocyte transgenic NOD mouse model, the 116C-NOD mouse.
Heat-killed Staphylococcus aureus reduces atherosclerosis by inducing anti-inflammatory macrophages.
Equilibrium of Treg/Th17 cells of peripheral blood in syphilitic patients with sero-resistance.
Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence.
p(⁷⁰S⁶K¹) in the TORC1 pathway is essential for the differentiation of Th17 Cells, but not Th1, Th2, or Treg cells in mice.
Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment.
Epidermal Notch1 recruits RORγ(+) group 3 innate lymphoid cells to orchestrate normal skin repair.
Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia.
Commensal enteric bacteria lipopolysaccharide impairs host defense against disseminated Candida albicans fungal infection.
Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut.
Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway.
CCR7-dependent trafficking of RORγ⁺ ILCs creates a unique microenvironment within mucosal draining lymph nodes.
T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate.
Estradiol inhibits Th17 cell differentiation through inhibition of RORγT transcription by recruiting the ERα/REA complex to estrogen response elements of the RORγT promoter.
Potential role of IL-17-producing iNKT cells in type 1 diabetes.
Myelin-specific T helper 17 cells promote adult hippocampal neurogenesis through indirect mechanisms.
The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor γt (RORγt) in Th17 cells.
The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells.
Self in vivo production of a synthetic biological drug CTLA4Ig using a minicircle vector.
Akt-dependent enhanced migratory capacity of Th17 cells from children with lupus nephritis.
High-dose interferon-γ promotes abortion in mice by suppressing Treg and Th17 polarization.
CLEC-2 is required for development and maintenance of lymph nodes.
Regulation of cyp26a1 on Th17 cells in mouse peri-implantation.
CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22.
CD5 enhances Th17-cell differentiation by regulating IFN-γ response and RORγt localization.
Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms.
Identification of transcriptional regulators in the mouse immune system.
CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium.
Interferon regulatory factor 3 controls interleukin-17 expression in CD8 T lymphocytes.
Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses.
Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells.
Peyer's patch inducer cells play a leading role in the formation of B and T cell zone architecture.
Mapping of NKp46(+) Cells in Healthy Human Lymphoid and Non-Lymphoid Tissues.
Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system.
Rank signaling links the development of invariant γδ T cell progenitors and Aire(+) medullary epithelium.
Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin.
TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B.
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.
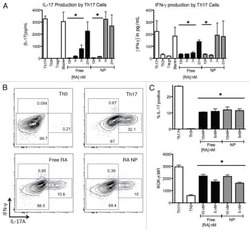
Development of a nanoparticulate formulation of retinoic acid that suppresses Th17 cells and upregulates regulatory T cells.
IL-1β-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells.
Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections.
Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis.
Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells.
Slominski AT, Brożyna AA, Kim TK, Elsayed MM, Janjetovic Z, Qayyum S, Slominski RM, Oak ASW, Li C, Podgorska E, Li W, Jetten AM, Tuckey RC, Tang EKY, Elmets C, Athar M
International journal of oncology 2022 Aug;61(2)
International journal of oncology 2022 Aug;61(2)
Iron controls T helper cell pathogenicity by promoting glucose metabolism in autoimmune myopathy.
Lai Y, Zhao S, Chen B, Huang Y, Guo C, Li M, Ye B, Wang S, Zhang H, Yang N
Clinical and translational medicine 2022 Aug;12(8):e999
Clinical and translational medicine 2022 Aug;12(8):e999
ILC3s control airway inflammation by limiting T cell responses to allergens and microbes.
Teng F, Tachó-Piñot R, Sung B, Farber DL, Worgall S, Hammad H, Lambrecht BN, Hepworth MR, Sonnenberg GF
Cell reports 2021 Nov 23;37(8):110051
Cell reports 2021 Nov 23;37(8):110051
AMPK induces regulatory innate lymphoid cells after traumatic brain injury.
Baban B, Braun M, Khodadadi H, Ward A, Alverson K, Malik A, Nguyen K, Nazarian S, Hess DC, Forseen S, Post AF, Vale FL, Vender JR, Hoda MN, Akbari O, Vaibhav K, Dhandapani KM
JCI insight 2021 Jan 11;6(1)
JCI insight 2021 Jan 11;6(1)
Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes.
Sano T, Kageyama T, Fang V, Kedmi R, Martinez CS, Talbot J, Chen A, Cabrera I, Gorshko O, Kurakake R, Yang Y, Ng C, Schwab SR, Littman DR
Cell reports 2021 Aug 24;36(8):109608
Cell reports 2021 Aug 24;36(8):109608
CD2(+) T-helper 17-like cells differentiated from a CD133(+) subpopulation of non-small cell lung carcinoma cells promote the growth of lung carcinoma.
Jia M, Jia X, Zhang D, Liu W, Yi S, Li Z, Cong B, Ma C, Li S, Zhang J
Annals of translational medicine 2021 Apr;9(8):687
Annals of translational medicine 2021 Apr;9(8):687
Abnormal Eating Patterns Cause Circadian Disruption and Promote Alcohol-Associated Colon Carcinogenesis.
Bishehsari F, Engen PA, Voigt RM, Swanson G, Shaikh M, Wilber S, Naqib A, Green SJ, Shetuni B, Forsyth CB, Saadalla A, Osman A, Hamaker BR, Keshavarzian A, Khazaie K
Cellular and molecular gastroenterology and hepatology 2020;9(2):219-237
Cellular and molecular gastroenterology and hepatology 2020;9(2):219-237
Intestinal vitamin D receptor knockout protects from oxazolone-induced colitis.
Shi Y, Liu Z, Cui X, Zhao Q, Liu T
Cell death & disease 2020 Jun 15;11(6):461
Cell death & disease 2020 Jun 15;11(6):461
Ahr-Foxp3-RORγt axis controls gut homing of CD4(+) T cells by regulating GPR15.
Xiong L, Dean JW, Fu Z, Oliff KN, Bostick JW, Ye J, Chen ZE, Mühlbauer M, Zhou L
Science immunology 2020 Jun 12;5(48)
Science immunology 2020 Jun 12;5(48)
Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer.
Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, Wang G, Tian Y, Ying J, Fan Z
Cell research 2020 Jul;30(7):610-622
Cell research 2020 Jul;30(7):610-622
JQ1, a bromodomain inhibitor, suppresses Th17 effectors by blocking p300-mediated acetylation of RORγt.
Wang X, Yang Y, Ren D, Xia Y, He W, Wu Q, Zhang J, Liu M, Du Y, Ren C, Li B, Shen J, Zhang Y
British journal of pharmacology 2020 Jul;177(13):2959-2973
British journal of pharmacology 2020 Jul;177(13):2959-2973
TSLP Produced by Aspergillus fumigatus-Stimulated DCs Promotes a Th17 Response Through the JAK/STAT Signaling Pathway in Fungal Keratitis.
Han F, Guo H, Wang L, Zhang Y, Sun L, Dai C, Wu X
Investigative ophthalmology & visual science 2020 Dec 1;61(14):24
Investigative ophthalmology & visual science 2020 Dec 1;61(14):24
BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis.
Jaén RI, Fernández-Velasco M, Terrón V, Sánchez-García S, Zaragoza C, Canales-Bueno N, Val-Blasco A, Vallejo-Cremades MT, Boscá L, Prieto P
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2020 Aug;34(8):10531-10546
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2020 Aug;34(8):10531-10546
The colonic macrophage transcription factor RBP-J orchestrates intestinal immunity against bacterial pathogens.
Kang L, Zhang X, Ji L, Kou T, Smith SM, Zhao B, Guo X, Pineda-Torra I, Wu L, Hu X
The Journal of experimental medicine 2020 Apr 6;217(4)
The Journal of experimental medicine 2020 Apr 6;217(4)
Protein arginine methyltransferase 5 promotes cholesterol biosynthesis-mediated Th17 responses and autoimmunity.
Webb LM, Sengupta S, Edell C, Piedra-Quintero ZL, Amici SA, Miranda JN, Bevins M, Kennemer A, Laliotis G, Tsichlis PN, Guerau-de-Arellano M
The Journal of clinical investigation 2020 Apr 1;130(4):1683-1698
The Journal of clinical investigation 2020 Apr 1;130(4):1683-1698
Establishment of Chronic Typhoid Infection in a Mouse Carriage Model Involves a Type 2 Immune Shift and T and B Cell Recruitment to the Gallbladder.
González JF, Kurtz J, Bauer DL, Hitt R, Fitch J, Wetzel A, La Perle K, White P, McLachlan J, Gunn JS
mBio 2019 Oct 1;10(5)
mBio 2019 Oct 1;10(5)
Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity.
Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, Fraser GL, Gallini Comeau CA, Glickman JN, Fuller MH, Layden BT, Garrett WS
Immunity 2019 Nov 19;51(5):871-884.e6
Immunity 2019 Nov 19;51(5):871-884.e6
BCAP links IL-1R to the PI3K-mTOR pathway and regulates pathogenic Th17 cell differentiation.
Deason K, Troutman TD, Jain A, Challa DK, Mandraju R, Brewer T, Ward ES, Pasare C
The Journal of experimental medicine 2018 Sep 3;215(9):2413-2428
The Journal of experimental medicine 2018 Sep 3;215(9):2413-2428
Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer.
Pennock ND, Martinson HA, Guo Q, Betts CB, Jindal S, Tsujikawa T, Coussens LM, Borges VF, Schedin P
Journal for immunotherapy of cancer 2018 Oct 1;6(1):98
Journal for immunotherapy of cancer 2018 Oct 1;6(1):98
Inflammation-induced Id2 promotes plasticity in regulatory T cells.
Hwang SM, Sharma G, Verma R, Byun S, Rudra D, Im SH
Nature communications 2018 Nov 9;9(1):4736
Nature communications 2018 Nov 9;9(1):4736
Human CD40 ligand-expressing type 3 innate lymphoid cells induce IL-10-producing immature transitional regulatory B cells.
Komlósi ZI, Kovács N, van de Veen W, Kirsch AI, Fahrner HB, Wawrzyniak M, Rebane A, Stanic B, Palomares O, Rückert B, Menz G, Akdis M, Losonczy G, Akdis CA
The Journal of allergy and clinical immunology 2018 Jul;142(1):178-194.e11
The Journal of allergy and clinical immunology 2018 Jul;142(1):178-194.e11
Trim33 mediates the proinflammatory function of Th17 cells.
Tanaka S, Jiang Y, Martinez GJ, Tanaka K, Yan X, Kurosaki T, Kaartinen V, Feng XH, Tian Q, Wang X, Dong C
The Journal of experimental medicine 2018 Jul 2;215(7):1853-1868
The Journal of experimental medicine 2018 Jul 2;215(7):1853-1868
Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation.
Emgård J, Kammoun H, García-Cassani B, Chesné J, Parigi SM, Jacob JM, Cheng HW, Evren E, Das S, Czarnewski P, Sleiers N, Melo-Gonzalez F, Kvedaraite E, Svensson M, Scandella E, Hepworth MR, Huber S, Ludewig B, Peduto L, Villablanca EJ, Veiga-Fernandes H, Pereira JP, Flavell RA, Willinger T
Immunity 2018 Jan 16;48(1):120-132.e8
Immunity 2018 Jan 16;48(1):120-132.e8
High dietary salt intake correlates with modulated Th17-Treg cell balance resulting in enhanced bone loss and impaired bone-microarchitecture in male mice.
Dar HY, Singh A, Shukla P, Anupam R, Mondal RK, Mishra PK, Srivastava RK
Scientific reports 2018 Feb 6;8(1):2503
Scientific reports 2018 Feb 6;8(1):2503
Fisetin inhibits pristine-induced systemic lupus erythematosus in a murine model through CXCLs regulation.
Xu SP, Li YS
International journal of molecular medicine 2018 Dec;42(6):3220-3230
International journal of molecular medicine 2018 Dec;42(6):3220-3230
Epigenetic activation during T helper 17 cell differentiation is mediated by Tripartite motif containing 28.
Jiang Y, Liu Y, Lu H, Sun SC, Jin W, Wang X, Dong C
Nature communications 2018 Apr 12;9(1):1424
Nature communications 2018 Apr 12;9(1):1424
Characterisation of innate lymphoid cell populations at different sites in mice with defective T cell immunity.
Dutton EE, Camelo A, Sleeman M, Herbst R, Carlesso G, Belz GT, Withers DR
Wellcome open research 2017;2:117
Wellcome open research 2017;2:117
JunB is essential for IL-23-dependent pathogenicity of Th17 cells.
Hasan Z, Koizumi SI, Sasaki D, Yamada H, Arakaki N, Fujihara Y, Okitsu S, Shirahata H, Ishikawa H
Nature communications 2017 May 30;8:15628
Nature communications 2017 May 30;8:15628
Cutting Edge: Selective Oral ROCK2 Inhibitor Reduces Clinical Scores in Patients with Psoriasis Vulgaris and Normalizes Skin Pathology via Concurrent Regulation of IL-17 and IL-10.
Zanin-Zhorov A, Weiss JM, Trzeciak A, Chen W, Zhang J, Nyuydzefe MS, Arencibia C, Polimera S, Schueller O, Fuentes-Duculan J, Bonifacio KM, Kunjravia N, Cueto I, Soung J, Fleischmann RM, Kivitz A, Lebwohl M, Nunez M, Woodson J, Smith SL, West RF, Berger M, Krueger JG, Ryan JL, Waksal SD
Journal of immunology (Baltimore, Md. : 1950) 2017 May 15;198(10):3809-3814
Journal of immunology (Baltimore, Md. : 1950) 2017 May 15;198(10):3809-3814
Protein kinase CK2 controls T-cell polarization through dendritic cell activation in response to contact sensitizers.
de Bourayne M, Gallais Y, El Ali Z, Rousseau P, Damiens MH, Cochet C, Filhol O, Chollet-Martin S, Pallardy M, Kerdine-Römer S
Journal of leukocyte biology 2017 Mar;101(3):703-715
Journal of leukocyte biology 2017 Mar;101(3):703-715
CD40-signalling abrogates induction of RORγt(+) Treg cells by intestinal CD103(+) DCs and causes fatal colitis.
Barthels C, Ogrinc A, Steyer V, Meier S, Simon F, Wimmer M, Blutke A, Straub T, Zimber-Strobl U, Lutgens E, Marconi P, Ohnmacht C, Garzetti D, Stecher B, Brocker T
Nature communications 2017 Mar 9;8:14715
Nature communications 2017 Mar 9;8:14715
WASH maintains NKp46(+) ILC3 cells by promoting AHR expression.
Xia P, Liu J, Wang S, Ye B, Du Y, Xiong Z, Han ZG, Tong L, Fan Z
Nature communications 2017 Jun 7;8:15685
Nature communications 2017 Jun 7;8:15685
High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice.
Wei Y, Lu C, Chen J, Cui G, Wang L, Yu T, Yang Y, Wu W, Ding Y, Li L, Uede T, Chen Z, Diao H
Oncotarget 2017 Jan 3;8(1):70-82
Oncotarget 2017 Jan 3;8(1):70-82
PRMT5-Selective Inhibitors Suppress Inflammatory T Cell Responses and Experimental Autoimmune Encephalomyelitis.
Webb LM, Amici SA, Jablonski KA, Savardekar H, Panfil AR, Li L, Zhou W, Peine K, Karkhanis V, Bachelder EM, Ainslie KM, Green PL, Li C, Baiocchi RA, Guerau-de-Arellano M
Journal of immunology (Baltimore, Md. : 1950) 2017 Feb 15;198(4):1439-1451
Journal of immunology (Baltimore, Md. : 1950) 2017 Feb 15;198(4):1439-1451
IL-1β, But Not Programed Death-1 and Programed Death Ligand Pathway, Is Critical for the Human Th17 Response to Mycobacterium tuberculosis.
Stephen-Victor E, Sharma VK, Das M, Karnam A, Saha C, Lecerf M, Galeotti C, Kaveri SV, Bayry J
Frontiers in immunology 2016;7:465
Frontiers in immunology 2016;7:465
An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells.
Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA
Nature medicine 2016 Sep;22(9):1013-22
Nature medicine 2016 Sep;22(9):1013-22
Human mast cells capture, store, and release bioactive, exogenous IL-17A.
Noordenbos T, Blijdorp I, Chen S, Stap J, Mul E, Cañete JD, Lubberts E, Yeremenko N, Baeten D
Journal of leukocyte biology 2016 Sep;100(3):453-62
Journal of leukocyte biology 2016 Sep;100(3):453-62
Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin α1β2 in Newly Formed Tertiary Lymphoid Structures.
Nayar S, Campos J, Chung MM, Navarro-Núñez L, Chachlani M, Steinthal N, Gardner DH, Rankin P, Cloake T, Caamaño JH, McGettrick HM, Watson SP, Luther S, Buckley CD, Barone F
Journal of immunology (Baltimore, Md. : 1950) 2016 Sep 1;197(5):1957-67
Journal of immunology (Baltimore, Md. : 1950) 2016 Sep 1;197(5):1957-67
Protein C receptor (PROCR) is a negative regulator of Th17 pathogenicity.
Kishi Y, Kondo T, Xiao S, Yosef N, Gaublomme J, Wu C, Wang C, Chihara N, Regev A, Joller N, Kuchroo VK
The Journal of experimental medicine 2016 Oct 17;213(11):2489-2501
The Journal of experimental medicine 2016 Oct 17;213(11):2489-2501
Development of Th17-Associated Interstitial Kidney Inflammation in Lupus-Prone Mice Lacking the Gene Encoding STAT-1.
Yiu G, Rasmussen TK, Ajami B, Haddon DJ, Chu AD, Tangsombatvisit S, Haynes WA, Diep V, Steinman L, Faix J, Utz PJ
Arthritis & rheumatology (Hoboken, N.J.) 2016 May;68(5):1233-44
Arthritis & rheumatology (Hoboken, N.J.) 2016 May;68(5):1233-44
ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer.
Wang J, Zou JX, Xue X, Cai D, Zhang Y, Duan Z, Xiang Q, Yang JC, Louie MC, Borowsky AD, Gao AC, Evans CP, Lam KS, Xu J, Kung HJ, Evans RM, Xu Y, Chen HW
Nature medicine 2016 May;22(5):488-96
Nature medicine 2016 May;22(5):488-96
B-cell anergy induces a Th17 shift in a novel B lymphocyte transgenic NOD mouse model, the 116C-NOD mouse.
Carrascal J, Carrillo J, Arpa B, Egia-Mendikute L, Rosell-Mases E, Pujol-Autonell I, Planas R, Mora C, Mauricio D, Ampudia RM, Vives-Pi M, Verdaguer J
European journal of immunology 2016 Mar;46(3):593-608
European journal of immunology 2016 Mar;46(3):593-608
Heat-killed Staphylococcus aureus reduces atherosclerosis by inducing anti-inflammatory macrophages.
Frodermann V, van Duijn J, van Puijvelde GH, van Santbrink PJ, Lagraauw HM, de Vries MR, Quax PH, Bot I, Foks AC, de Jager SC, Kuiper J
Journal of internal medicine 2016 Jun;279(6):592-605
Journal of internal medicine 2016 Jun;279(6):592-605
Equilibrium of Treg/Th17 cells of peripheral blood in syphilitic patients with sero-resistance.
Zhao J, Ma J, Zhang X, Li Q, Yang X
Experimental and therapeutic medicine 2016 Jun;11(6):2300-2304
Experimental and therapeutic medicine 2016 Jun;11(6):2300-2304
Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence.
Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, Eberl G, Grice EA, Veiga-Fernandes H
Nature 2016 Jul 21;535(7612):440-443
Nature 2016 Jul 21;535(7612):440-443
p(⁷⁰S⁶K¹) in the TORC1 pathway is essential for the differentiation of Th17 Cells, but not Th1, Th2, or Treg cells in mice.
Sasaki CY, Chen G, Munk R, Eitan E, Martindale J, Longo DL, Ghosh P
European journal of immunology 2016 Jan;46(1):212-22
European journal of immunology 2016 Jan;46(1):212-22
Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment.
Zhong C, Cui K, Wilhelm C, Hu G, Mao K, Belkaid Y, Zhao K, Zhu J
Nature immunology 2016 Feb;17(2):169-78
Nature immunology 2016 Feb;17(2):169-78
Epidermal Notch1 recruits RORγ(+) group 3 innate lymphoid cells to orchestrate normal skin repair.
Li Z, Hodgkinson T, Gothard EJ, Boroumand S, Lamb R, Cummins I, Narang P, Sawtell A, Coles J, Leonov G, Reboldi A, Buckley CD, Cupedo T, Siebel C, Bayat A, Coles MC, Ambler CA
Nature communications 2016 Apr 21;7:11394
Nature communications 2016 Apr 21;7:11394
Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia.
Nanjappa SG, Hernández-Santos N, Galles K, Wüthrich M, Suresh M, Klein BS
PLoS pathogens 2015 Sep;11(9):e1005161
PLoS pathogens 2015 Sep;11(9):e1005161
Commensal enteric bacteria lipopolysaccharide impairs host defense against disseminated Candida albicans fungal infection.
Jiang TT, Chaturvedi V, Ertelt JM, Xin L, Clark DR, Kinder JM, Way SS
Mucosal immunology 2015 Jul;8(4):886-95
Mucosal immunology 2015 Jul;8(4):886-95
Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut.
Kim MH, Taparowsky EJ, Kim CH
Immunity 2015 Jul 21;43(1):107-19
Immunity 2015 Jul 21;43(1):107-19
Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway.
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH
Mucosal immunology 2015 Jan;8(1):80-93
Mucosal immunology 2015 Jan;8(1):80-93
CCR7-dependent trafficking of RORγ⁺ ILCs creates a unique microenvironment within mucosal draining lymph nodes.
Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, Filbey KJ, Maizels RM, Hepworth MR, Sonnenberg GF, Milling S, Withers DR
Nature communications 2015 Jan 9;6:5862
Nature communications 2015 Jan 9;6:5862
T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate.
Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR
Immunity 2015 Dec 15;43(6):1101-11
Immunity 2015 Dec 15;43(6):1101-11
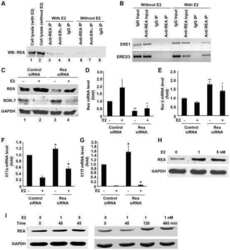
Estradiol inhibits Th17 cell differentiation through inhibition of RORγT transcription by recruiting the ERα/REA complex to estrogen response elements of the RORγT promoter.
Chen RY, Fan YM, Zhang Q, Liu S, Li Q, Ke GL, Li C, You Z
Journal of immunology (Baltimore, Md. : 1950) 2015 Apr 15;194(8):4019-28
Journal of immunology (Baltimore, Md. : 1950) 2015 Apr 15;194(8):4019-28
Potential role of IL-17-producing iNKT cells in type 1 diabetes.
Li S, Joseph C, Becourt C, Klibi J, Luce S, Dubois-Laforgue D, Larger E, Boitard C, Benlagha K
PloS one 2014;9(4):e96151
PloS one 2014;9(4):e96151
Myelin-specific T helper 17 cells promote adult hippocampal neurogenesis through indirect mechanisms.
Niebling J, E Rünker A, Schallenberg S, Kretschmer K, Kempermann G
F1000Research 2014;3:169
F1000Research 2014;3:169
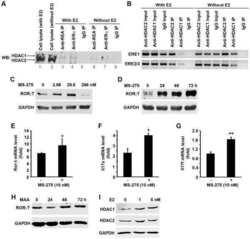
The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor γt (RORγt) in Th17 cells.
Han L, Yang J, Wang X, Wu Q, Yin S, Li Z, Zhang J, Xing Y, Chen Z, Tsun A, Li D, Piccioni M, Zhang Y, Guo Q, Jiang L, Bao L, Lv L, Li B
The Journal of biological chemistry 2014 Sep 12;289(37):25546-55
The Journal of biological chemistry 2014 Sep 12;289(37):25546-55
The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells.
Tang W, Wang H, Claudio E, Tassi I, Ha HL, Saret S, Siebenlist U
Immunity 2014 Oct 16;41(4):555-66
Immunity 2014 Oct 16;41(4):555-66
Self in vivo production of a synthetic biological drug CTLA4Ig using a minicircle vector.
Rim YA, Yi H, Kim Y, Park N, Jung H, Kim J, Jung SM, Park SH, Ju JH
Scientific reports 2014 Nov 6;4:6935
Scientific reports 2014 Nov 6;4:6935
Akt-dependent enhanced migratory capacity of Th17 cells from children with lupus nephritis.
Kshirsagar S, Riedl M, Billing H, Tönshoff B, Thangavadivel S, Steuber C, Staude H, Wechselberger G, Edelbauer M
Journal of immunology (Baltimore, Md. : 1950) 2014 Nov 15;193(10):4895-903
Journal of immunology (Baltimore, Md. : 1950) 2014 Nov 15;193(10):4895-903
High-dose interferon-γ promotes abortion in mice by suppressing Treg and Th17 polarization.
Liu HY, Liu ZK, Chao H, Li Z, Song Z, Yang Y, Peng JP
Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 2014 May;34(5):394-403
Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 2014 May;34(5):394-403
CLEC-2 is required for development and maintenance of lymph nodes.
Bénézech C, Nayar S, Finney BA, Withers DR, Lowe K, Desanti GE, Marriott CL, Watson SP, Caamaño JH, Buckley CD, Barone F
Blood 2014 May 15;123(20):3200-7
Blood 2014 May 15;123(20):3200-7
Regulation of cyp26a1 on Th17 cells in mouse peri-implantation.
Liu HY, Chao H, Liu ZK, Xia HF, Song Z, Yang Y, Peng JP
Journal of cellular and molecular medicine 2014 Mar;18(3):455-67
Journal of cellular and molecular medicine 2014 Mar;18(3):455-67
CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22.
Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR
The Journal of experimental medicine 2014 Jul 28;211(8):1571-83
The Journal of experimental medicine 2014 Jul 28;211(8):1571-83
CD5 enhances Th17-cell differentiation by regulating IFN-γ response and RORγt localization.
McGuire DJ, Rowse AL, Li H, Peng BJ, Sestero CM, Cashman KS, De Sarno P, Raman C
European journal of immunology 2014 Apr;44(4):1137-42
European journal of immunology 2014 Apr;44(4):1137-42
Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms.
Xiao S, Yosef N, Yang J, Wang Y, Zhou L, Zhu C, Wu C, Baloglu E, Schmidt D, Ramesh R, Lobera M, Sundrud MS, Tsai PY, Xiang Z, Wang J, Xu Y, Lin X, Kretschmer K, Rahl PB, Young RA, Zhong Z, Hafler DA, Regev A, Ghosh S, Marson A, Kuchroo VK
Immunity 2014 Apr 17;40(4):477-89
Immunity 2014 Apr 17;40(4):477-89
Identification of transcriptional regulators in the mouse immune system.
Jojic V, Shay T, Sylvia K, Zuk O, Sun X, Kang J, Regev A, Koller D, Immunological Genome Project Consortium, Best AJ, Knell J, Goldrath A, Joic V, Koller D, Shay T, Regev A, Cohen N, Brennan P, Brenner M, Kim F, Rao TN, Wagers A, Heng T, Ericson J, Rothamel K, Ortiz-Lopez A, Mathis D, Benoist C, Bezman NA, Sun JC, Min-Oo G, Kim CC, Lanier LL, Miller J, Brown B, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Monach P, Blair DA, Dustin ML, Shinton SA, Hardy RR, Laidlaw D, Collins J, Gazit R, Rossi DJ, Malhotra N, Sylvia K, Kang J, Kreslavsky T, Fletcher A, Elpek K, Bellemarte-Pelletier A, Malhotra D, Turley S
Nature immunology 2013 Jun;14(6):633-43
Nature immunology 2013 Jun;14(6):633-43
CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium.
Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, Niess JH
Mucosal immunology 2013 Jan;6(1):177-88
Mucosal immunology 2013 Jan;6(1):177-88
Interferon regulatory factor 3 controls interleukin-17 expression in CD8 T lymphocytes.
Ysebrant de Lendonck L, Tonon S, Nguyen M, Vandevenne P, Welsby I, Martinet V, Molle C, Charbonnier LM, Leo O, Goriely S
Proceedings of the National Academy of Sciences of the United States of America 2013 Aug 20;110(34):E3189-97
Proceedings of the National Academy of Sciences of the United States of America 2013 Aug 20;110(34):E3189-97
Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses.
Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B
Nature immunology 2013 Apr;14(4):372-9
Nature immunology 2013 Apr;14(4):372-9
Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA
Nature 2013 Apr 25;496(7446):518-22
Nature 2013 Apr 25;496(7446):518-22
Peyer's patch inducer cells play a leading role in the formation of B and T cell zone architecture.
Nakagawa R, Togawa A, Nagasawa T, Nishikawa S
Journal of immunology (Baltimore, Md. : 1950) 2013 Apr 1;190(7):3309-18
Journal of immunology (Baltimore, Md. : 1950) 2013 Apr 1;190(7):3309-18
Mapping of NKp46(+) Cells in Healthy Human Lymphoid and Non-Lymphoid Tissues.
Tomasello E, Yessaad N, Gregoire E, Hudspeth K, Luci C, Mavilio D, Hardwigsen J, Vivier E
Frontiers in immunology 2012;3:344
Frontiers in immunology 2012;3:344
Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system.
Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lécuyer MA, Ifergan I, Viel É, Bourbonnière L, Beauseigle D, Terouz S, Hachehouche L, Gendron S, Poirier J, Jobin C, Duquette P, Flanagan K, Yednock T, Arbour N, Prat A
Brain : a journal of neurology 2012 Oct;135(Pt 10):2906-24
Brain : a journal of neurology 2012 Oct;135(Pt 10):2906-24
Rank signaling links the development of invariant γδ T cell progenitors and Aire(+) medullary epithelium.
Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, Paolino M, Penninger JM, Simon AK, Nitta T, Ohigashi I, Takahama Y, Caamano JH, Hayday AC, Lane PJ, Jenkinson EJ, Anderson G
Immunity 2012 Mar 23;36(3):427-37
Immunity 2012 Mar 23;36(3):427-37
Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin.
Ichiyama K, Sekiya T, Inoue N, Tamiya T, Kashiwagi I, Kimura A, Morita R, Muto G, Shichita T, Takahashi R, Yoshimura A
Immunity 2011 May 27;34(5):741-54
Immunity 2011 May 27;34(5):741-54
TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B.
Gaddis DE, Michalek SM, Katz J
Journal of immunology (Baltimore, Md. : 1950) 2011 May 15;186(10):5772-83
Journal of immunology (Baltimore, Md. : 1950) 2011 May 15;186(10):5772-83
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.
El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A
Nature immunology 2011 Jun;12(6):568-75
Nature immunology 2011 Jun;12(6):568-75
Development of a nanoparticulate formulation of retinoic acid that suppresses Th17 cells and upregulates regulatory T cells.
Capurso NA, Look M, Jeanbart L, Nowyhed H, Abraham C, Craft J, Fahmy TM
Self/nonself 2010 Oct;1(4):335-340
Self/nonself 2010 Oct;1(4):335-340
IL-1β-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells.
Li L, Kim J, Boussiotis VA
Journal of immunology (Baltimore, Md. : 1950) 2010 Oct 1;185(7):4148-53
Journal of immunology (Baltimore, Md. : 1950) 2010 Oct 1;185(7):4148-53
Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections.
Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, Ho L, Sauer JD, Iwakura Y, Metzger DW, Monack DM
Journal of immunology (Baltimore, Md. : 1950) 2010 Apr 1;184(7):3755-67
Journal of immunology (Baltimore, Md. : 1950) 2010 Apr 1;184(7):3755-67
Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis.
Poffenberger MC, Straka N, El Warry N, Fang D, Shanina I, Horwitz MS
PloS one 2009 Jul 9;4(7):e6207
PloS one 2009 Jul 9;4(7):e6207
Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells.
Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R, Rolink AG, Finke D
Journal of immunology (Baltimore, Md. : 1950) 2009 Aug 15;183(4):2217-21
Journal of immunology (Baltimore, Md. : 1950) 2009 Aug 15;183(4):2217-21
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
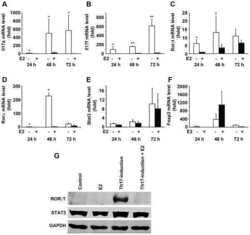
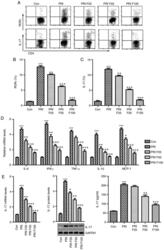
- Figure 6 Splenic Th17 cells and pro-inflammatory cytokine expression. The lymphocytes were isolated from mice subjected to various treatments. (A) Flow cytometry was used to evaluate RORgammat + CD3 + CD4 + Th17 cells among splenic CD4 + cells obtained from mice. Quantification of (B) RORgamma and (C) IL-17 from the flow cytometry results. (D) The mRNA levels of the cytokines IL-6, IFN-gamma, TNF-alpha, IL-1beta, and MCP-1beta in the spleen tissue samples were assessed. (E) IL-17 mRNA and protein levels were evaluated by reverse transcription-quantitative polymerase chain reaction, western blot and ELISA. Values are expressed as the mean +- standard error of the mean (n=10 in each group). *** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
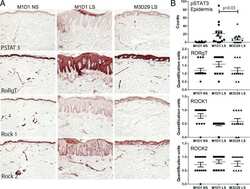
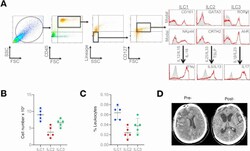
- Figure 1 Presence and frequency of ILC subtypes within the meninges of severe TBI patients. ( A ) Dura was collected from consecutive, severe TBI patients undergoing decompressive craniectomy to alleviate elevated intracranial pressure. ILCs were sorted using forward scatter (FSC)/side scatter (SSC) and identified as CD45 + , lineage-negative (Lin - ), CD127 + lymphoid cells. ILCs subtypes were further defined as ILC1:, CD45 + Lin - CD127 + CD161 + NKp44 + ; ILC2, CD45 + Lin - CD127 + GATA3 + CRTH2 + ; and ILC3, CD45 + Lin - CD127 + RORgammat + AhR + , as shown in representative flow cytometry scatterplots. Gray shaded areas indicate isotype controls. To demonstrate functionality, ILCs were further stimulated with cytokine cocktails, and production of signature cytokines was assessed (ILC1, IFN-gamma; ILC2, IL-5/IL-13; ILC3, IL-17). ( B and C ) Frequency of ILC subtypes from individual patients, expressed as total cell number ( B ) and % leukocytes ( C ) ( n = 5). Scatterplots depict mean +- SD. ( D ) Computed tomography scan of a TBI patient before (Pre-) and after (Post-) decompressive craniectomy surgery. The dura was collected during surgery at the time of bone flap removal.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
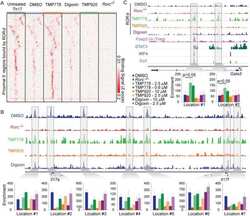
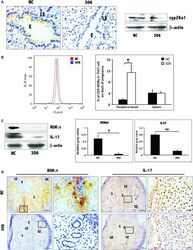
- Further certainty, the regulation of cyp26a1 on Th17 cells at uterine implantation sites by uterine horn injection of anti-cyp26a1 antibody during mice peri-implantation. ( A ) Decrease in cyp26a1 levels at D5 uterine implantation sites following uterine horn injection of cyp26a1 antibody on D3 of pregnancy. Cyp26a1 levels were reduced at uterine implantation sites based on Western blotting and immunohistochemistry. beta-actin was used as a loading control. The bars represent the SD of the mean of the relative value in grey (cyp26a1/beta-actin). A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05, ** P < 0.01; bars = 50 mum. The data were derived from three separate samples from pregnant mice. At least, three independent experiments were repeated for this time-point. A total of nine samples from pregnant mice were assessed. ( B ) Th17cell increased in the peripheral blood and the spleen following uterine horn injection of cyp26a1 antibody. Flow cytometry histogram of Th17 cells is on the left side. Th17 cells are CD 4 + ROR gammat + . Th17 cell ratio was evaluated by flow cytometry analysis. The data are expressed as the% of CD 4 + ROR gammat + double positive cells. The bars represent the SD of the mean. A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05. Three independent experiments were repeated for this time-point.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- 7 Inhibition of CK2 activity modulates mRNA expression of IRFs in MoDCs. Immature MoDCs were pretreated for 1 h with DMSO 0.1% or CX-4945 (CX, 5 uM) and then stimulated for 6 h with (A) NiSO 4 500 uM or (B) DNCB 10 uM. IRF-1, -8, and -4 mRNAs were quantified by real-time RT-PCR. Naive CD4 + T lymphocytes were coincubated with NiSO4-stimulated MoDCs (C) in the absence or presence of CX-4945 (5 uM). On day 5, cells were restimulated with anti-CD3/CD2/CD28 beads for 48 h and stained with CD4-Alexa Fluor 700, CD3-FITC, RorgammaT-APC, GATA3-PE, and T-Bet Brilliant Violet 605. Living cells (ZombieAqua negative cells) were analyzed by flow cytometry. Data represent the results of 5 independent experiments and are normalized to the control. * P < 0.05; ** P < 0.01 (Mann-Whitney test).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4. Flow cytometric mapping of the expression of forkhead box P3 and retinoic acid-related orphan receptor gammat (ROR-gammat) molecules in CD4 + cells of peripheral blood.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
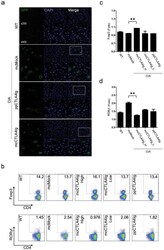
- Figure 5 Successful transfection of mcCTLA4Ig into hepatocytes. (a) GFP expression of mcMock plasmids in CIA mice liver is shown as positive fluorescence. The minicircular form showed a relatively high positive expression in hepatocytes compared to the parental form. (b) Splenocytes of CIA were analyzed by flow cytometry. (c) mcCTLA4Ig-treated mice had more Foxp3 + cells compared with mice given a low dose of mcCTLA4Ig or ppCTLA4Ig. (d) Fewer RORgammat + cells were seen in mice given mcCTLA4Ig compared with those given mcMock, low-dose mcCTLA4Ig, or ppCTLA4Ig. (** P < 0.005 ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
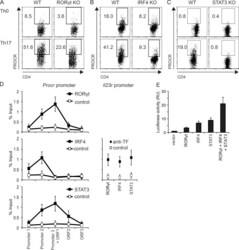
- Figure 2. Rorgammat, IRF4, and STAT3 bind the Procr promoter and induce PROCR expression. Naive CD4 + T cells from Rorgammat KO mice (A), CD4 Cre xIRF4 fl/fl mice (B), or CD4 Cre xSTAT3 fl/fl mice (C) and control mice were polarized under Th17 conditions (TGF-beta1 and IL-6) or without cytokines (Th0) as control. On day 3, PROCR expression was determined by flow cytometry. (D) Purified WT naive CD4 + T cells were differentiated into Th17 cells in the presence of TGF-beta1 and IL-6. After 4 d, binding of Rorgammat, IRF4, or STAT3 to the Procr and IL23r promoters was assessed by ChIP-PCR (TF, transcription factor; mean +- SD of triplicates). (E) Procr promoter activity was measured in HEK293T cells transfected with a luciferase vector driven by 1,040 bp of the Procr promoter (pGL4-Procr), and the indicated transcription factor expression constructs (mean +- SD of triplicates). Representative plots of two to three independent experiments are shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
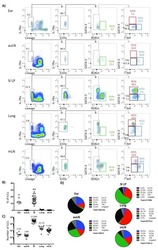
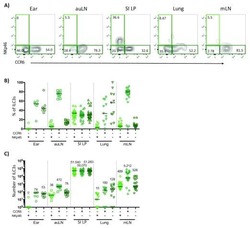
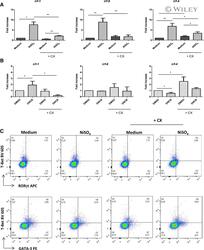
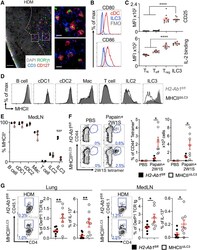
- Figure 2. ILC3s limit CD4 + T cell responses to allergens in the airway (A) Representative immunofluorescence staining for ILC3s and T cells in the MedLNs of HDM-exposed mice (representative of 2 independent experiments and n = 3 mice per group). Images taken at 20x magnification; scale bars, 50 mum (left panel) and 10 mum (inserts; right panel). Dashed line indicates the B cell follicle border. (B) Histograms demonstrating CD80 and CD86 expression in MedLN cells following HDM exposure (representative of 2 independent assays containing 8 mice each). FMO, fluorescence minus one. (C) Graphs quantifying CD25 staining or IL-2 binding in gated MedLN cells following HDM exposure (pooled from 2 independent assays, n = 10). Naive T cells were gated as CD44 - CD62L + , effector T cells were gated as CD44 + CD62L - , and Tregs were gated as FoxP3 + . (D and E) Representative plots of MHC class II expression in designated immune cells (D) and quantification of MHC class II staining on those cell types in the MedLN following HDM exposure (E) (representative of 2 independent assays, n = 4). (F) Representative plots and quantitative graphs of the frequency and numbers of 2W1S-specific CD4 + T cells in lung parenchyma from H2-Ab1 fl/fl control mice and MHC class II DeltaILC3 mice after exposure to papain plus 2W1S peptide or PBS control. Cells were gated on live CD45 + CD19 - TCRbeta + CD4 + (data pooled from 2 independent assays). (G) Representative plots and quantitative graphs of the fre
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
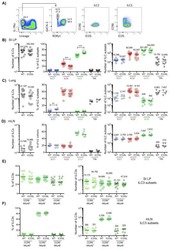
- Figure 3 Interleukin (IL)-22 expression is reduced in Citrobacter rodentium -infected CX 3 CR1 GFP/GFP animals. ( a ) From the proximal colon of noninfected and C. rodentium -infected CX 3 CR1 GFP/GFP and wild-type littermate controls mRNA was isolated. cDNA was prepared by reverse transcription and quantitative real-time PCR was performed with cyber green and the indicated primers. beta-actin was used as a housekeeping gene to normalize cDNA input between samples. Normalized ct-values of the untreated samples (baseline) are set to 1 and values are plotted as fold expression of the baseline. The assays were carried out in triplicates. As per indicated, group 4 animals were analyzed. ( b ) IL-22 expression by colonic lamina propria (cLP) cell isolates from noninfected and C. rodentium -infected B6, (age- and sex-matched) and CX 3 CR1 GFP/+ and CX 3 CR1 GFP/GFP animals was analyzed by multicolor flow cytometry. Numbers indicate the percentage of IL-22-positive cells. ( c ) cLP IL22 + cells from noninfected and C. rodentium- infected B6, (age- and sex-matched), and CX 3 CR1 GFP/+ and CX 3 CR1 GFP/GFP animals were stained for CD3e and CD4. Numbers indicate the percentage of CD3e + , CD3e + CD4 + , CD4 + , and CD3e - CD4 - cells within the IL-22 + cell population. Data from an individual representative mouse per group (of five to seven individual mice analyzed per group) are shown. ( d ) cLP IL-22 + CD3e - CD4 + cells were (intracellular) stained for related orphan receptor gamma-
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
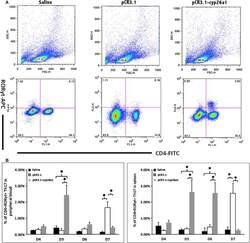
- Figure 2 Th17 cell increased in the peripheral blood and the spleen following pCR3.1-cyp26a1 immunization during mice peri-implantation, especially on D5 of mice pregnancy. (A) Flow cytometry scatterplot of Th17 cells. Th17 cells are CD4 + RORgammat + . (B) The Th17 cell proportion increased significantly in the peripheral blood and the spleen under the influence of recombinant plasmid, especially on D5 of pregnancy. The CD4 + RORgammat + Th17 cell ratio was evaluated by flow cytometry analysis. The data are expressed as the% of CD4 + RORgammat + double positive cells. The bars represent the SD of the mean. A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05. At least, three independent experiments were repeated for each time-point. A total of 49 samples from pregnant mice were assessed.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
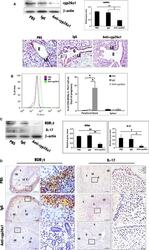
- Figure 3 Decrease in Th17 cells at uterine implantation sites following pCR3.1-cyp26a1 immunization during mice peri-implantation. (A) Th17 levels were significantly reduced at uterine implantation sites based on Western blotting, especially on D5 of mice pregnancy. RORgammat is the specific marker of Th17 cell, IL-17 is the specific cytokines secreted by Th17 cells. beta-actin was used as a loading control. The bars represent the SD of the mean of the relative value in grey (RORgammat/beta-actin or IL-17/beta-actin). A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05, ** P < 0.01. At least, three independent experiments were repeated for each time-point. A total of 36 samples from pregnant mice were assessed. (B) Th17 levels were obviously reduced at implantation sites of D5 pregnancy based on RORgammat and IL-17 immunohistochemistry stain; bars = 200 and 25 mum. At least, three independent experiments were repeated for this time-point. A total of nine samples from pregnant mice were assessed. E, embryo; S, uterine stroma; LE, Luminal epithelium; GE, Glandular epithelium.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 4 Further certainty, the regulation of cyp26a1 on Th17 cells at uterine implantation sites by uterine horn injection of anti-cyp26a1 antibody during mice peri-implantation. (A) Decrease in cyp26a1 levels at D5 uterine implantation sites following uterine horn injection of cyp26a1 antibody on D3 of pregnancy. Cyp26a1 levels were reduced at uterine implantation sites based on Western blotting and immunohistochemistry. beta-actin was used as a loading control. The bars represent the SD of the mean of the relative value in grey (cyp26a1/beta-actin). A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05, ** P < 0.01; bars = 50 mum. The data were derived from three separate samples from pregnant mice. At least, three independent experiments were repeated for this time-point. A total of nine samples from pregnant mice were assessed. (B) Th17cell increased in the peripheral blood and the spleen following uterine horn injection of cyp26a1 antibody. Flow cytometry histogram of Th17 cells is on the left side. Th17 cells are CD4 + RORgammat + . Th17 cell ratio was evaluated by flow cytometry analysis. The data are expressed as the% of CD4 + RORgammat + double positive cells. The bars represent the SD of the mean. A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05. Three independent experiments were repeated for this time-point
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 5 Further certainty, the regulation of cyp26a1 on Th17 cells at uterine implantation sites by tail vein injection of cyp26a1 lentiviral interference segment (No. 306) during mice peri-implantation. (A) Decrease in cyp26a1 levels at uterine implantation sites of D5 following tail vein injection of cyp26a1 lentiviral interference segment (306) on D3 of pregnancy. Cyp26a1 levels were reduced at uterine implantation sites based on Western blotting and immunohistochemistry; bars = 25 mum. The data were derived from two separate samples from pregnant mice. At least, three independent experiments were repeated for this time-point. A total of six samples from pregnant mice were assessed. (B) Th17 cell increased in the peripheral blood, but the spleen following tail vein injection of cyp26a1 lentiviral interference segment (No. 306). Flow cytometry histogram of Th17 cells is on the left side. Th17 cells are CD4 + RORgammat + . Th17 cell ratio was evaluated by flow cytometry analysis. The data are expressed as the% of CD4 + RORgammat + double positive cells. The bars represent the SD of the mean. A paired t -test was used to assess the significance of differences. Pairwise comparisons between each treatment group, * P < 0.05. Three independent experiments were repeated. A total of nine samples from pregnant mice were assessed. (C) Th17 levels were significantly reduced at uterine implantation sites based on Western blotting of D5 pregnancy. beta-actin was used as a loading contr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 7 Increase in RARalpha at uterine implantation sites following pCR3.1-cyp26a1 immunization during mice peri-implantation and further certainty, the influence of RARalpha on Th17 cells at uterine implantation sites by tail vein injection of RARalpha specific agonist and antagonist during mice peri-implantation. (A) RARalpha levels were significantly increased at uterine implantation sites based on Western blotting. beta-actin was used as a loading control. (B) RARalpha levels were obviously increased at implantation sites of D5 pregnancy based on immunohistochemistry following pCR3.1-cyp26a1 immunization; bars = 200 and 25 mum. The data were derived from three separate samples from pregnant mice. At least, three independent experiments were repeated for this time-point. A total of nine samples from pregnant mice were assessed. E, embryo; S, uterine stroma; LE, Luminal epithelium; GE, Glandular epithelium. (C) RARalpha and RORgammat protein expression changes in uterus on D5 of pregnancy in response to administration of agonist (Am-580) and antagonist (Ro-41-5253) of RARalpha. RARalpha levels were significantly increased by the activation of Am-580 and significantly reduced by the inhibition of Ro-41-5253 at uterine implantation sites based on western blotting of pregnancy D5. RORgammat levels just took the opposite changes on RARalpha. beta-actin was used as a loading control. The administration (125 mug/mouse) of RARalpha agonist (Am-580) or antagonist (Ro-41-5253) was
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
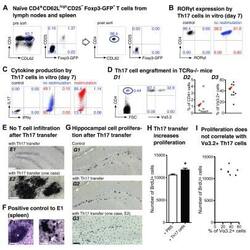
- Figure 2. Th17-polarized CD4 + T cells are sufficient to promote hippocampal precursor cell proliferation in adult TCRalpha -/- mice. ( A - C ) Th17 polarization in vitro . ( A ) Flow cytometry of encephalitogenic CD4 + T cells. Dot plots show pre-sort (left) and post-sort (right) analysis of naive T cells (CD4 + CD62L high CD25 - Foxp3 GFP- ) from pooled spleen and lymph nodes of 2D2 x Foxp3 GFP mice. FACS-purified T cell populations were cultured under Th17-polarizing conditions, as described in the Methods section. On day 7, efficiency of Th17 cell differentiation was confirmed by intracellular flow cytometry of ( B ) the Th17 transcription factor ROR-gammat and ( C ) the Th17 and Th1 signature cytokines IL-17 and IFN-gamma, respectively. ( D - I ) Impact of adoptive Th17 cell transfer on hippocampal precursor cell proliferation in TCRalpha -/- mice. ( D ) On day 7, 4 x 10 6 total cells from Th17 polarization cultures were injected i.v. into adult TCRalpha -/- mice. (D1) Dot plots show representative flow cytometry of CD4 + T cells in peripheral blood of recipient mice that express the Valpha3.2 subunit of the transgenic 2D2 TCR, two weeks after adoptive transfer. (D2) and (D3) Graphs show composite percentages of total CD4 + T cells (D2) and MOG 35-55 -reactive Valpha3.2 + T cells among gated CD4 + T cell populations (D3) from peripheral blood of recipient mice. The arrowheads in (D2) and (D3) highlight an individual mouse that exhibited immune cell infiltrations in the b
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
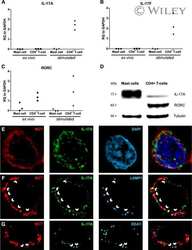
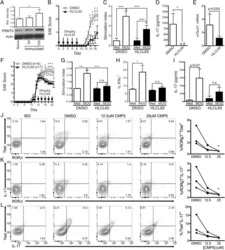
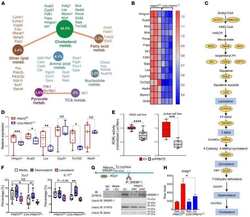
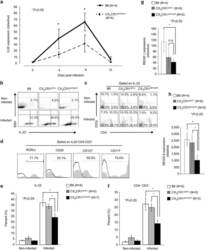
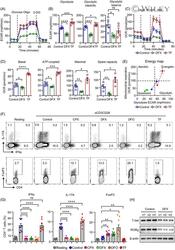
- 2 FIGURE Iron chelation inhibited metabolic activities and modulated the differentiation of CD4 + T-cells. (A)-(E) CD4 + T-cells isolated from healthy controls (HC) were stimulated with anti-CD3/CD28 beads for 3 d. Ciclopirox (CPX) (1 muM), deferasirox (DFX) (2 muM), deferoxamine (DFO) (2 muM) and transferrin (TF, 50 mug/ml) were included in some of the experiments as indicated. For metabolic activities evaluation of CD4 + T-cells, Glycolysis Stress Test Kit and Mito Stress Test Kit were used to test extracellular acidification rate (ECAR) and oxygen consumption rate (OCR), respectively, by a Seahorse XF96 analyser. (A and B) Glucose, oligomycin (Oligo) and 2-deoxyglucose (2-DG) were injected sequentially and ECAR were recorded. ECAR tracing curves and parameters of glycolysis, glycolysis capacity and glycolysis reserve were summarized ( n = 5). (C and D) Oligo, carbonyl cyanide- p -trifluoromethoxyphenylhydrazone (FCCP) and rotenone/antimycin A (R/A) were injected sequentially. OCR of basal respiration, respiration coupled to ATP production, maximal respiration and respiratory spare capacity were summarized from four independent samples. (E) Energy map showing metabolic potential of CD4 + T-cells. (F and G) CD4 + T-cells were stimulated with anti-CD3/CD28 beads in the presence of CPX (1 muM), DFX (2 muM), DFO (2 muM) and TF (50 mug/ml) for 5 d. CD4 + T-cells were stained with Brilliant Violet 421-conjugated anti-interferon gamma (IFNgamma), APC-conjugated anti-interleukin (I
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
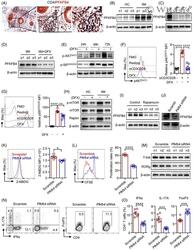
- 5 FIGURE Iron promotes glucose metabolism in CD4 + T-cells through PFKFB4 in idiopathic inflammatory myopathies (IIM). (A) Double staining of CD4 (dark blue) and PFKFB4 (red) of skin sections from patients with IIM by immunohistochemistry. Original magnification: 200x. (B)-(I) CD4 + T-cells were stimulated with anti-CD3/CD28 beads for 72 h in the presence of ciclopirox (CPX) (1 muM), deferasirox (DFX) (2 muM), deferoxamine (DFO) (2 muM) or rapamycin (10 nM) as indicated. PFKFB4 expression in CD4 + T-cells from IIM patients (p) or healthy controls (HC) ( n ) (B), or CD4 + T-cells from HC treated with iron chelators (C), or CD4 + T-cells from IIM treated with DFX (D) was measured by western blot. Representative bands of three independent samples. (E) Phosphorylation of AKT (p-AKT) and p-S6 (ribosomal protein) expression in CD4 + T-cells from HC was measured by western blot. (F and G) CD4 + T-cells from HC were stained with primary antibodies against p-AKT and p-S6, followed by Alexa Fluor 594-conjugated goat anti-rabbit IgG and measured by flow cytometry ( n = 6). (H) Expression of phosphorylation of mTOR (p-mTOR), mTOR and Raptor in CD4 + T-cells from IIM or HC treated with or without DFX was measured by western blot. Representative bands of three independent samples. (I) PFKFB4 expression in CD4 + T-cells from HC treated with rapamycin was measured by western blot ( n = 3). (J)-(O) CD4 + T-cells from HC or IIM were electrotransfected with Pfkfb4 - or scramble siRNA. (J) Pfkfb
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
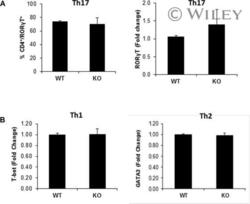
- Effect of p70 S6K1 on the status of transcription factors involved in the differentiation of Th1, Th2, and Th17 cells. Naive splenic CD4 + T cells (CD4 + CD62L + ) from WT and p70 S6K1 KO mice were activated by plate-bound anti-CD3 and anti-CD28 under either Th1, Th2, or Th17 skewing conditions. (A) Flow cytometry and RT-PCR analyses were performed for retinoic acid receptor-related orphan receptor gamma T (RORgammaT) using in vitro-differentiated Th17 cells. Following in vitro differentiation, Th17 cells were stimulated with PMA and ionomycin for 4 h, last 2 h with monensin. Stimulated cells were either stained with antibodies against CD4 and RORgammaT and analyzed by flow cytometry (left), or used for total RNA isolation (right). The cDNA was then analyzed by quantitative PCR to measure RORgammaT transcript levels (right). These experiments were first normalized to a housekeeping gene (either beta actin or GAPDH). Then the normalized results were compared to wild-type Th17 cells to determine the fold change. (B) RT-PCR analyses of T-bet (left) and GATA3 (right) levels were done using in vitro-differentiated Th1 and Th2 cells, respectively. The amount of T-bet and GATA3 mRNA expression in WT and KO differentiated cells were normalized first to beta actin, and then the normalized values were compared to wild type to determine the fold change. All data shown as mean + SEM from two independent experiments with total six samples per group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
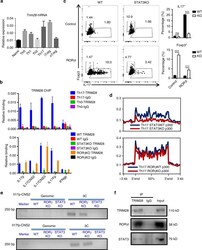
- Fig. 7 Cytokine signaling regulated TRIM28 recruitment. a Expression of TRIM28 in different T-cell subsets and naive CD4 + T cells. b TRIM28 ChIP-qPCR results in WT Th0, WT Th17, STAT3 KO Th17 or RORgammat KO Th17 cells. c RORgammat overexpression in WT or STAT3 KO CD4 + T cells polarized under neutral (Th0) condition. d Overlay of p300 binding peaks in WT/STAT3KO(Upper) or WT/RORgammaKO (lower) Th17 cells over Th17-specific SEs with decreased p300 intensity. e 3C-PCR experiments were performed in WT and TRIM28KO Th17 cells cultured for 3 days and the PCR products were acquired by nest PCR. M is short for marker, and the whole DNA gels are shown in Supplementary Fig. 8 . f Immunoblot results of TRIM28 immunoprecipitated protein complexes as detected by RORgammat and STAT3 antibodies in Th17 cells. The whole WB gels are shown in Supplementary Fig. 9 . Those experiments were repeated twice with consistent results, and Student's t test was used for the statistical test (ns = not significant; * p < 0.05, ** p < 0.01, *** p < 0.005). All error bars represent SDs
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
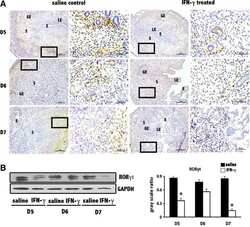
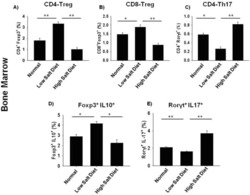
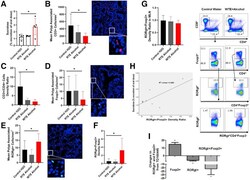
- Figure 6 Rest-time food and alcohol access promote tumor-associated inflammation. ( A ) Colon permeability increased in WTE + alcohol mice using urine excretion ratios of sucralose. ( B and C ) By using immunofluorescence, ( B ) polyp-associated CD3 + cell density per surface area as well as ( C ) the CD3 + /CD4 + cell density ratio was determined for each polyp and compared between the groups. ( D and E ) The density of ( D ) FOXP3 + and ( E ) RORgammat + cells in the peripolyp surface areas was lower and higher, respectively, in the WTE + alcohol mice vs the control water group (N = 99 polyp images were used for quantification). Representative stained slide images are shown for ( B ) CD3, ( D ) FOXP3, and ( E ) RORgammat at 20x magnification, with the inset at 40x magnification. Between-group comparisons of the RORgammat + /FOXP3 + ratio in the ( F ) mucosa and ( G ) mesenteric lymph nodes as determined by flow cytometry are shown. Representative gating scheme of 1 mouse for CD4 + T-cell intracellular FOXP3 and RORgammat cytometry is shown. No significant differences in FOXP3 + and RORgammat + T-cell abundance were observed among the tested groups. ( H ) Correlation between the urine excretion ratio of sucralose and the polyp-associated RORgammat + /FOXP3 + ratio. ( I ) TD160445 treatment increased mucosal FOXP3 + and decreased RORgammat + cell density, as well as the RORgammat/FOXP3 + ratio in WTE + alcohol-treated mice (N = 48 polyp images were used for t
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
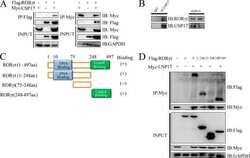
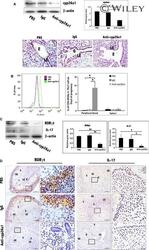
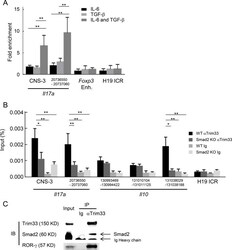
- Figure 7. Cooperative regulation of IL-17 and IL-10 by Trim33, Smad2, and ROR-gamma. (A) Trim33 binding was assessed by ChIP-PCR analysis. PCR amplification was conducted with input DNA and I.P. DNA prepared from chromatin precipitated with anti-Trim33 antibodies, with C57BL/6 CD4 + T cells stimulated by anti-CD3 and anti-CD28 antibodies under indicated conditions. Primer for the H19 ICR locus was used as a negative control. Data are representative of three independent experiments. (B) Trim33 ChIP was performed with WT and Smad2 KO CD4 + T cells cultured under Th17 skewing conditions. (C) Physical association of Trim33 with Smad2 and ROR-gamma in Th17 cells. Lysates of Th17 cells were subjected to immunoprecipitation with anti-Trim33 antibody or isotype Ig, followed by immunoblot analysis with anti-Trim33, Smad2, and ROR-gamma antibodies. Data are representative of four independent experiments. Error bars show means +- SD. *, P < 0.05; **, P < 0.01.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunoprecipitation
Immunoprecipitation Other assay
Other assay