Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA5-27126 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- BLZF1 Monoclonal Antibody (OTI8E8)
- Antibody type
- Monoclonal
- Antigen
- Recombinant protein fragment
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- OTI8E8
- Vial size
- 100 μL
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Tankyrase-1-mediated degradation of Golgin45 regulates glycosyltransferase trafficking and protein glycosylation in Rab2-GTP-dependent manner.
Yue X, Tiwari N, Zhu L, Ngo HDT, Lim JM, Gim B, Jing S, Wang Y, Qian Y, Lee I
Communications biology 2021 Dec 7;4(1):1370
Communications biology 2021 Dec 7;4(1):1370
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
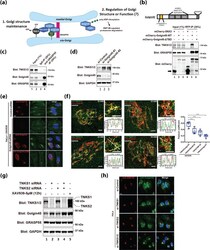
- Experimental details
- Fig. 1 TNKS1 targeting to the Golgi membranes is mediated by Golgin45 and localizes to the cis- and medial-Golgi cisternae. a A brief overview of Golgin45 protein complexes. Golgin45 forms a multi-protein complex with GRASP55 and ACBD3 that contributes to Golgi structure maintenance. Golgin45 was identified as a substrate for Tankyrase1-dependent PARylation and subsequent proteasomal degradation. The physiological role of TNKS1-Golgin45 interaction is currently unknown. b Golgin45 binding to TNKS1 is dependent on Tankyrase-binding domain (TBD). The protein extracts from HeLa cells transfected with mCherry tagged Sorting Nexin 3 (mCherry-SNX3 as a control), mCherry-Golgin45 or mCherry-Golgin45-DeltaTBD (deletion of TBD at the Golgin45 N-terminal domain) were immunoprecipitated with anti-RFP agarose beads. These lysates and the immunoprecipitates (anti-RFP IPs) were analyzed by western blotting using anti-mCherry antibody and antibodies against the indicated proteins. Deletion of TBD at the Golgin45 N-terminal domain abrogates its interaction with endogenous TNKS1. Experiments were repeated three times and representative western blots are shown here. c Immunoprecipitation experiments showing that endogenous GRASP55 selectively forms a complex with Golgin45, but not with TNKS1/2, suggesting that TNKS1/2 are excluded from GRASP55-Golgin45 complex. The protein extracts from HeLa cells were immunoprecipitated with anti-GRASP55 or normal rabbit IgG. These lysates and the immunopreci
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Other assay
Other assay