MA3-921
antibody from Invitrogen Antibodies
Targeting: CACNA2D1
CACNA2, CACNL2A, LINC01112, lncRNA-N3, MHS3
Antibody data
- Antibody Data
- Antigen structure
- References [54]
- Comments [0]
- Validations
- Immunocytochemistry [6]
- Immunohistochemistry [2]
- Flow cytometry [6]
- Other assay [8]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-921 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CACNA2D1 Monoclonal Antibody (20A)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA3-921 detects 1,4-dihydropyridine (DHP) receptor alpha-2 subunit from human, rat, mouse, guinea pig and rabbit skeletal and cardiac muscle. MA3-921 has been successfully used in Western blot, immunocytochemistry, immunofluorescence, and immunohistochemistry procedures. By Western blot, this antibody detects a 220 kDa protein under non-reducing and a 143 kDa protein under reducing conditions representing DHP from rabbit skeletal muscle membrane preparations. Immunohistochemical staining of DHP in rabbit skeletal muscle with MA3-921 results in double rows of discrete punctate staining representing pairs of triads on the opposing sides of the Z-lines. The MA3-921 antigen is purified rabbit DHP receptor.
- Reactivity
- Human, Mouse, Rat, Guinea Pig, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- 20A
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Effects of Topical Gabapentin on Ocular Pain and Tear Secretion.
Sarcolipin haploinsufficiency prevents dystrophic cardiomyopathy in mdx mice.
MicroRNA-107 inhibits proliferation and invasion of laryngeal squamous cell carcinoma cells by targeting CACNA2D1 in vitro.
A homozygous missense variant in CACNB4 encoding the auxiliary calcium channel beta4 subunit causes a severe neurodevelopmental disorder and impairs channel and non-channel functions.
α2δ1 may be a potential marker for cancer stem cell in laryngeal squamous cell carcinoma.
SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR.
Fluorescence Resonance Energy Transfer-based Structural Analysis of the Dihydropyridine Receptor α1S Subunit Reveals Conformational Differences Induced by Binding of the β1a Subunit.
Cannabinoid signalling inhibits sarcoplasmic Ca(2+) release and regulates excitation-contraction coupling in mammalian skeletal muscle.
Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function.
Amino acid residues 489-503 of dihydropyridine receptor (DHPR) β1a subunit are critical for structural communication between the skeletal muscle DHPR complex and type 1 ryanodine receptor.
Complete atrial-specific knockout of sodium-calcium exchange eliminates sinoatrial node pacemaker activity.
Tail-anchored membrane protein SLMAP is a novel regulator of cardiac function at the sarcoplasmic reticulum.
Kir2.6 regulates the surface expression of Kir2.x inward rectifier potassium channels.
Functional expression of transgenic 1sDHPR channels in adult mammalian skeletal muscle fibres.
Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure.
Reduced gain of excitation-contraction coupling in triadin-null myotubes is mediated by the disruption of FKBP12/RyR1 interaction.
Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure.
Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout.
Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development.
Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice.
Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle.
Slowing of cardiomyocyte Ca2+ release and contraction during heart failure progression in postinfarction mice.
S100A1 deficiency results in prolonged ventricular repolarization in response to sympathetic activation.
Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility.
Increased cardiomyocyte function and Ca2+ transients in mice during early congestive heart failure.
Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease.
Microarray profiling of skeletal muscle sarcoplasmic reticulum proteins.
Reduced expression of regucalcin in young and aged mdx diaphragm indicates abnormal cytosolic calcium handling in dystrophin-deficient muscle.
Daily exercise-induced cardioprotection is associated with changes in calcium regulatory proteins in hypertensive rats.
Increased sensitivity of the ryanodine receptor to halothane-induced oligomerization in malignant hyperthermia-susceptible human skeletal muscle.
Subcellular localization of the delayed rectifier K(+) channels KCNQ1 and ERG1 in the rat heart.
Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy.
Transgenic overexpression of the Ca2+-binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance.
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Ca2+ regulatory systems in rat myocardium are altered by 24 weeks treadmill training.
Use of continuous-elution gel electrophoresis as a preparative tool for blot overlay analysis.
Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle.
Calcium channel alpha(2)delta subunits-structure and Gabapentin binding.
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Effects of chronic low-frequency stimulation on Ca2+-regulatory membrane proteins in rabbit fast muscle.
Effects of chronic low-frequency stimulation on Ca2+-regulatory membrane proteins in rabbit fast muscle.
Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle.
Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle.
Alpha 1 and alpha 2 Ca2+ channel subunit expression in human neuronal and small cell carcinoma cells.
Localization of the alpha 1 and alpha 2 subunits of the dihydropyridine receptor and ankyrin in skeletal muscle triads.
The alpha 1 and alpha 2 polypeptides of the dihydropyridine-sensitive calcium channel differ in developmental expression and tissue distribution.
Cammalleri M, Amato R, Olivieri M, Pezzino S, Bagnoli P, Dal Monte M, Rusciano D
Frontiers in pharmacology 2021;12:671238
Frontiers in pharmacology 2021;12:671238
Sarcolipin haploinsufficiency prevents dystrophic cardiomyopathy in mdx mice.
Mareedu S, Pachon R, Thilagavathi J, Fefelova N, Balakrishnan R, Niranjan N, Xie LH, Babu GJ
American journal of physiology. Heart and circulatory physiology 2021 Jan 1;320(1):H200-H210
American journal of physiology. Heart and circulatory physiology 2021 Jan 1;320(1):H200-H210
MicroRNA-107 inhibits proliferation and invasion of laryngeal squamous cell carcinoma cells by targeting CACNA2D1 in vitro.
Huang C, Wang Z, Zhang K, Dong Y, Zhang A, Lu C, Liu L
Anti-cancer drugs 2020 Mar;31(3):260-271
Anti-cancer drugs 2020 Mar;31(3):260-271
A homozygous missense variant in CACNB4 encoding the auxiliary calcium channel beta4 subunit causes a severe neurodevelopmental disorder and impairs channel and non-channel functions.
Coste de Bagneaux P, von Elsner L, Bierhals T, Campiglio M, Johannsen J, Obermair GJ, Hempel M, Flucher BE, Kutsche K
PLoS genetics 2020 Mar;16(3):e1008625
PLoS genetics 2020 Mar;16(3):e1008625
α2δ1 may be a potential marker for cancer stem cell in laryngeal squamous cell carcinoma.
Huang C, Li Y, Zhao W, Zhang A, Lu C, Wang Z, Liu L
Cancer biomarkers : section A of Disease markers 2019;24(1):97-107
Cancer biomarkers : section A of Disease markers 2019;24(1):97-107
SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR.
Kim TY, Terentyeva R, Roder KH, Li W, Liu M, Greener I, Hamilton S, Polina I, Murphy KR, Clements RT, Dudley SC Jr, Koren G, Choi BR, Terentyev D
Cardiovascular research 2017 Mar 1;113(3):343-353
Cardiovascular research 2017 Mar 1;113(3):343-353
Fluorescence Resonance Energy Transfer-based Structural Analysis of the Dihydropyridine Receptor α1S Subunit Reveals Conformational Differences Induced by Binding of the β1a Subunit.
Mahalingam M, Perez CF, Fessenden JD
The Journal of biological chemistry 2016 Jun 24;291(26):13762-70
The Journal of biological chemistry 2016 Jun 24;291(26):13762-70
Cannabinoid signalling inhibits sarcoplasmic Ca(2+) release and regulates excitation-contraction coupling in mammalian skeletal muscle.
Oláh T, Bodnár D, Tóth A, Vincze J, Fodor J, Reischl B, Kovács A, Ruzsnavszky O, Dienes B, Szentesi P, Friedrich O, Csernoch L
The Journal of physiology 2016 Dec 15;594(24):7381-7398
The Journal of physiology 2016 Dec 15;594(24):7381-7398
Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function.
Sharma P, Abbasi C, Lazic S, Teng ACT, Wang D, Dubois N, Ignatchenko V, Wong V, Liu J, Araki T, Tiburcy M, Ackerley C, Zimmermann WH, Hamilton R, Sun Y, Liu PP, Keller G, Stagljar I, Scott IC, Kislinger T, Gramolini AO
Nature communications 2015 Sep 25;6:8391
Nature communications 2015 Sep 25;6:8391
Amino acid residues 489-503 of dihydropyridine receptor (DHPR) β1a subunit are critical for structural communication between the skeletal muscle DHPR complex and type 1 ryanodine receptor.
Eltit JM, Franzini-Armstrong C, Perez CF
The Journal of biological chemistry 2014 Dec 26;289(52):36116-24
The Journal of biological chemistry 2014 Dec 26;289(52):36116-24
Complete atrial-specific knockout of sodium-calcium exchange eliminates sinoatrial node pacemaker activity.
Groenke S, Larson ED, Alber S, Zhang R, Lamp ST, Ren X, Nakano H, Jordan MC, Karagueuzian HS, Roos KP, Nakano A, Proenza C, Philipson KD, Goldhaber JI
PloS one 2013;8(11):e81633
PloS one 2013;8(11):e81633
Tail-anchored membrane protein SLMAP is a novel regulator of cardiac function at the sarcoplasmic reticulum.
Nader M, Westendorp B, Hawari O, Salih M, Stewart AF, Leenen FH, Tuana BS
American journal of physiology. Heart and circulatory physiology 2012 Mar 1;302(5):H1138-45
American journal of physiology. Heart and circulatory physiology 2012 Mar 1;302(5):H1138-45
Kir2.6 regulates the surface expression of Kir2.x inward rectifier potassium channels.
Dassau L, Conti LR, Radeke CM, Ptáček LJ, Vandenberg CA
The Journal of biological chemistry 2011 Mar 18;286(11):9526-41
The Journal of biological chemistry 2011 Mar 18;286(11):9526-41
Functional expression of transgenic 1sDHPR channels in adult mammalian skeletal muscle fibres.
DiFranco M, Tran P, Quiñonez M, Vergara JL
The Journal of physiology 2011 Mar 15;589(Pt 6):1421-42
The Journal of physiology 2011 Mar 15;589(Pt 6):1421-42
Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure.
Al Moamen NJ, Prasad V, Bodi I, Miller ML, Neiman ML, Lasko VM, Alper SL, Wieczorek DF, Lorenz JN, Shull GE
Journal of molecular and cellular cardiology 2011 Jan;50(1):137-46
Journal of molecular and cellular cardiology 2011 Jan;50(1):137-46
Reduced gain of excitation-contraction coupling in triadin-null myotubes is mediated by the disruption of FKBP12/RyR1 interaction.
Eltit JM, Szpyt J, Li H, Allen PD, Perez CF
Cell calcium 2011 Feb;49(2):128-35
Cell calcium 2011 Feb;49(2):128-35
Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure.
Bueno CR Jr, Ferreira JC, Pereira MG, Bacurau AV, Brum PC
Journal of applied physiology (Bethesda, Md. : 1985) 2010 Sep;109(3):702-9
Journal of applied physiology (Bethesda, Md. : 1985) 2010 Sep;109(3):702-9
Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout.
Louch WE, Hougen K, Mørk HK, Swift F, Aronsen JM, Sjaastad I, Reims HM, Roald B, Andersson KB, Christensen G, Sejersted OM
The Journal of physiology 2010 Feb 1;588(Pt 3):465-78
The Journal of physiology 2010 Feb 1;588(Pt 3):465-78
Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development.
Grajales L, García J, Banach K, Geenen DL
Journal of molecular and cellular cardiology 2010 Apr;48(4):735-45
Journal of molecular and cellular cardiology 2010 Apr;48(4):735-45
Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice.
Ferreira JC, Bacurau AV, Bueno CR Jr, Cunha TC, Tanaka LY, Jardim MA, Ramires PR, Brum PC
Experimental biology and medicine (Maywood, N.J.) 2010 Apr;235(4):497-505
Experimental biology and medicine (Maywood, N.J.) 2010 Apr;235(4):497-505
Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle.
O'Connell K, Ohlendieck K
Proteomics 2009 Dec;9(24):5509-24
Proteomics 2009 Dec;9(24):5509-24
Slowing of cardiomyocyte Ca2+ release and contraction during heart failure progression in postinfarction mice.
Mørk HK, Sjaastad I, Sejersted OM, Louch WE
American journal of physiology. Heart and circulatory physiology 2009 Apr;296(4):H1069-79
American journal of physiology. Heart and circulatory physiology 2009 Apr;296(4):H1069-79
S100A1 deficiency results in prolonged ventricular repolarization in response to sympathetic activation.
Ackermann GE, Domenighetti AA, Deten A, Bonath I, Marenholz I, Pedrazzini T, Erne P, Heizmann CW
General physiology and biophysics 2008 Jun;27(2):127-42
General physiology and biophysics 2008 Jun;27(2):127-42
Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility.
Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU
Endocrinology 2008 Feb;149(2):558-64
Endocrinology 2008 Feb;149(2):558-64
Increased cardiomyocyte function and Ca2+ transients in mice during early congestive heart failure.
Mørk HK, Sjaastad I, Sande JB, Periasamy M, Sejersted OM, Louch WE
Journal of molecular and cellular cardiology 2007 Aug;43(2):177-86
Journal of molecular and cellular cardiology 2007 Aug;43(2):177-86
Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease.
Hirata H, Watanabe T, Hatakeyama J, Sprague SM, Saint-Amant L, Nagashima A, Cui WW, Zhou W, Kuwada JY
Development (Cambridge, England) 2007 Aug;134(15):2771-81
Development (Cambridge, England) 2007 Aug;134(15):2771-81
Microarray profiling of skeletal muscle sarcoplasmic reticulum proteins.
Schulz JS, Palmer N, Steckelberg J, Jones SJ, Zeece MG
Biochimica et biophysica acta 2006 Sep;1764(9):1429-35
Biochimica et biophysica acta 2006 Sep;1764(9):1429-35
Reduced expression of regucalcin in young and aged mdx diaphragm indicates abnormal cytosolic calcium handling in dystrophin-deficient muscle.
Doran P, Dowling P, Donoghue P, Buffini M, Ohlendieck K
Biochimica et biophysica acta 2006 Apr;1764(4):773-85
Biochimica et biophysica acta 2006 Apr;1764(4):773-85
Daily exercise-induced cardioprotection is associated with changes in calcium regulatory proteins in hypertensive rats.
Collins HL, Loka AM, Dicarlo SE
American journal of physiology. Heart and circulatory physiology 2005 Feb;288(2):H532-40
American journal of physiology. Heart and circulatory physiology 2005 Feb;288(2):H532-40
Increased sensitivity of the ryanodine receptor to halothane-induced oligomerization in malignant hyperthermia-susceptible human skeletal muscle.
Glover L, Heffron JJ, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2004 Jan;96(1):11-8
Journal of applied physiology (Bethesda, Md. : 1985) 2004 Jan;96(1):11-8
Subcellular localization of the delayed rectifier K(+) channels KCNQ1 and ERG1 in the rat heart.
Rasmussen HB, Møller M, Knaus HG, Jensen BS, Olesen SP, Jørgensen NK
American journal of physiology. Heart and circulatory physiology 2004 Apr;286(4):H1300-9
American journal of physiology. Heart and circulatory physiology 2004 Apr;286(4):H1300-9
Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy.
Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N
Journal of molecular biology 2003 Sep 5;332(1):171-82
Journal of molecular biology 2003 Sep 5;332(1):171-82
Transgenic overexpression of the Ca2+-binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance.
Most P, Remppis A, Pleger ST, Löffler E, Ehlermann P, Bernotat J, Kleuss C, Heierhorst J, Ruiz P, Witt H, Karczewski P, Mao L, Rockman HA, Duncan SJ, Katus HA, Koch WJ
The Journal of biological chemistry 2003 Sep 5;278(36):33809-17
The Journal of biological chemistry 2003 Sep 5;278(36):33809-17
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Dowling P, Lohan J, Ohlendieck K
European journal of cell biology 2003 May;82(5):222-30
European journal of cell biology 2003 May;82(5):222-30
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Dowling P, Lohan J, Ohlendieck K
European journal of cell biology 2003 May;82(5):222-30
European journal of cell biology 2003 May;82(5):222-30
Ca2+ regulatory systems in rat myocardium are altered by 24 weeks treadmill training.
Morán M, Saborido A, Megías A
Pflugers Archiv : European journal of physiology 2003 May;446(2):161-8
Pflugers Archiv : European journal of physiology 2003 May;446(2):161-8
Use of continuous-elution gel electrophoresis as a preparative tool for blot overlay analysis.
Mulvey C, Ohlendieck K
Analytical biochemistry 2003 Aug 1;319(1):122-30
Analytical biochemistry 2003 Aug 1;319(1):122-30
Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle.
Culligan K, Banville N, Dowling P, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Feb;92(2):435-45
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Feb;92(2):435-45
Calcium channel alpha(2)delta subunits-structure and Gabapentin binding.
Marais E, Klugbauer N, Hofmann F
Molecular pharmacology 2001 May;59(5):1243-8
Molecular pharmacology 2001 May;59(5):1243-8
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Harmon S, Froemming GR, Leisner E, Pette D, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Harmon S, Froemming GR, Leisner E, Pette D, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Froemming GR, Ohlendieck K
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Froemming GR, Ohlendieck K
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Glover L, Culligan K, Cala S, Mulvey C, Ohlendieck K
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Glover L, Culligan K, Cala S, Mulvey C, Ohlendieck K
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Effects of chronic low-frequency stimulation on Ca2+-regulatory membrane proteins in rabbit fast muscle.
Ohlendieck K, Frömming GR, Murray BE, Maguire PB, Leisner E, Traub I, Pette D
Pflugers Archiv : European journal of physiology 1999 Oct;438(5):700-8
Pflugers Archiv : European journal of physiology 1999 Oct;438(5):700-8
Effects of chronic low-frequency stimulation on Ca2+-regulatory membrane proteins in rabbit fast muscle.
Ohlendieck K, Frömming GR, Murray BE, Maguire PB, Leisner E, Traub I, Pette D
Pflugers Archiv : European journal of physiology 1999 Oct;438(5):700-8
Pflugers Archiv : European journal of physiology 1999 Oct;438(5):700-8
Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle.
Protasi F, Franzini-Armstrong C, Allen PD
The Journal of cell biology 1998 Feb 23;140(4):831-42
The Journal of cell biology 1998 Feb 23;140(4):831-42
Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle.
Protasi F, Franzini-Armstrong C, Allen PD
The Journal of cell biology 1998 Feb 23;140(4):831-42
The Journal of cell biology 1998 Feb 23;140(4):831-42
Alpha 1 and alpha 2 Ca2+ channel subunit expression in human neuronal and small cell carcinoma cells.
Morton ME, Cassidy TN, Froehner SC, Gilmour BP, Laurens RL
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1994 Aug;8(11):884-8
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1994 Aug;8(11):884-8
Localization of the alpha 1 and alpha 2 subunits of the dihydropyridine receptor and ankyrin in skeletal muscle triads.
Flucher BE, Morton ME, Froehner SC, Daniels MP
Neuron 1990 Sep;5(3):339-51
Neuron 1990 Sep;5(3):339-51
The alpha 1 and alpha 2 polypeptides of the dihydropyridine-sensitive calcium channel differ in developmental expression and tissue distribution.
Morton ME, Froehner SC
Neuron 1989 May;2(5):1499-506
Neuron 1989 May;2(5):1499-506
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
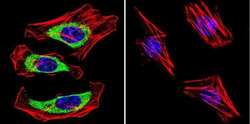
- Experimental details
- Immunofluorescent analysis of Dihydropyridine Receptor alpha-2 in HeLa Cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Dihydropyridine Receptor alpha-2 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
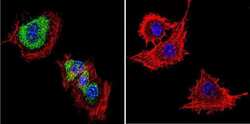
- Experimental details
- Immunofluorescent analysis of Dihydropyridine Receptor alpha-2 in PC12 Cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:20 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Dihydropyridine Receptor alpha-2 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
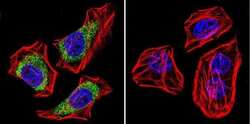
- Experimental details
- Immunofluorescent analysis of Dihydropyridine Receptor alpha-2 in U251 Cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Dihydropyridine Receptor alpha-2 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
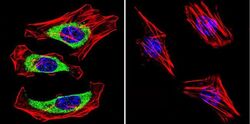
- Experimental details
- Immunofluorescent analysis of Dihydropyridine Receptor alpha-2 in HeLa Cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Dihydropyridine Receptor alpha-2 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
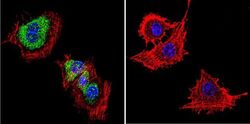
- Experimental details
- Immunofluorescent analysis of Dihydropyridine Receptor alpha-2 in PC12 Cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:20 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Dihydropyridine Receptor alpha-2 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
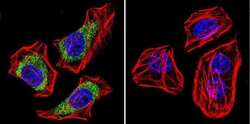
- Experimental details
- Immunofluorescent analysis of Dihydropyridine Receptor alpha-2 in U251 Cells. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 overnight at 4 C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503). Dihydropyridine Receptor alpha-2 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Images were taken at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
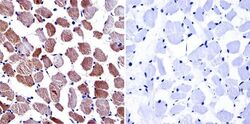
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized human skeletal muscle tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a Mouse Monoclonal Antibody recognizing Dihydropyridine Receptor alpha-2 (Product # MA3-921) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
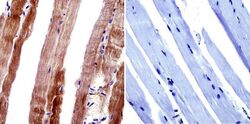
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized mouse skeletal muscle tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a Mouse Monoclonal Antibody recognizing Dihydropyridine Receptor alpha-2 (Product # MA3-921) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
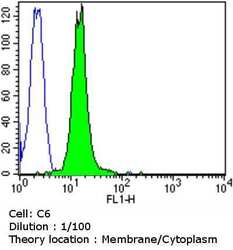
- Experimental details
- Flow cytometry analysis of Dihydropyridine Receptor alpha-2 in C6 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
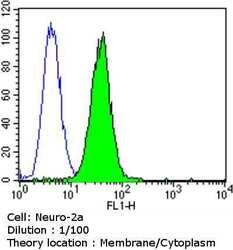
- Experimental details
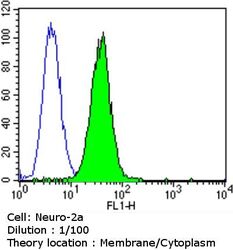
- Flow cytometry analysis of Dihydropyridine Receptor alpha-2 in Neuro-2a cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
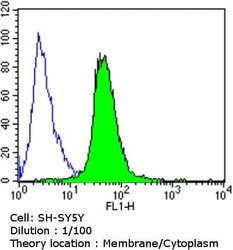
- Experimental details
- Flow cytometry analysis of Dihydropyridine Receptor alpha-2 in SH-SY5Y cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
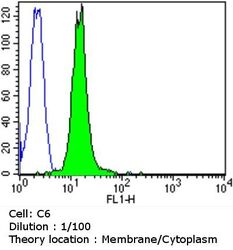
- Experimental details
- Flow cytometry analysis of Dihydropyridine Receptor alpha-2 in C6 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Flow cytometry analysis of Dihydropyridine Receptor alpha-2 in Neuro-2a cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
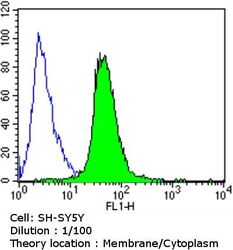
- Experimental details
- Flow cytometry analysis of Dihydropyridine Receptor alpha-2 in SH-SY5Y cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 min at room temperature and incubated with a Dihydropyridine Receptor alpha-2 monoclonal antibody (Product # MA3-921) at a dilution of 1:100 for 60 min at room temperature. Cells were then incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
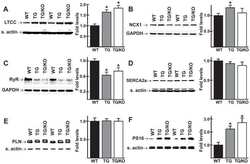
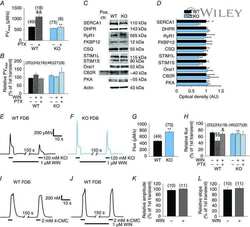
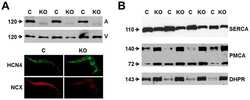
- Figure 1 Expression of NCX1 and other Ca handling proteins in atrial-specific NCX1 knockout mice. A . Immunoblot (top) of mouse atrial (A) and ventricular (V) homogenate using an antibody to NCX1 in wildtype control (C) and atrial-specific NCX1 KO mice. There is complete absence of NCX1 protein in the immunoblot. The new band in the KO lanes at ~110 kDa represents nonfunctional NCX in KO atria after excision of exon 11 by Cre recombinase [13] . Ventricular expression of NCX1 is unaffected in atrial-specific KO mice. The bottom panel shows immunofluorescence of isolated SAN node myocytes from control (C) and KO hearts. Myocytes were co-immunolabled with antibodies against HCN4 and NCX1. Both control and KO SAN cells stained positive for HCN4, but only control SAN cells showed staining of NCX at the membrane. B . Immunoblots of sarcoendoplasmic reticulum Ca ATPase (SERCA), plasma membrane Ca pump (PMCA; the lower band at 72 kDa represents an active proteolytic fragment), and dihydropyridine receptor (DHPR) in control (C) and NCX1 KO atria. Note the reduction in SERCA and the increase in PMCA and DHPR in KO.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
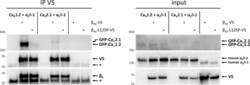
- Fig 8 The p.Leu125Pro mutation impairs complex formation of beta 4b with P/Q-type Ca V 2.1 and L-type Ca V 1.2 channels. HEK293T cells were co-transfected with beta 4b -V5 or beta 4b -L125P-V5 expression construct, mouse alpha2delta-1 expression construct and GFP-Ca v 1.2 or GFP-Ca v 2.1 (alpha 1 subunits) expression construct as indicated. beta 4b -V5 or beta 4b -L125P-V5 was immunoprecipitated with V5-coupled Protein G dynabeads. As control, lysates from cells only transfected with beta 4b -V5 or beta 4b -L125P-V5 construct were incubated with an anti-normal mouse IgG antibody coupled to Dynabeads. Co-precipitated GFP-tagged alpha 1 subunit was detected by anti-GFP antibody. beta 4b -V5 proteins were detected by anti-V5-HRP or anti-beta 4 antibody. Ectopically expressed (mouse) and endogenous (human) alpha2delta-1 were detected by anti-alpha2delta-1 antibody (input, right panel). The band at ~55 kDa corresponds to the heavy chain of the IgG antibody and is marked by an asterisk in the immunoblots of the co-immunoprecipitation (IP V5; left panel). A representative blot of four (Ca V 1.2) and three (Ca V 2.1) independent experiments is shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
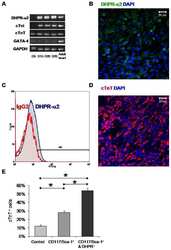
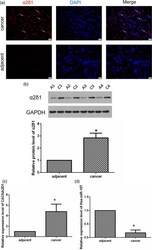
- Fig. 1 CACNA2D1 and miR-107 are inversely correlated in tissues. (a) This is an immunofluorescence photograph of a LSCC patient (T4N2M0, moderately differentiated, x400). The arrow shows the typical alpha2delta1 expression (red). (b) The images of alpha2delta1 protein expression detected by WB in adjacent normal tissues and LSCC tissues are as follows: A1 represents the adjacent tissue from patient No.1; C1 represents the LSCC tissue from patient No.1; A2 represents the adjacent tissue from patient No.2; and C2 represents the LSCC tissue from patient No. 2, and so on. * P < 0.05. (c and d) qPCR detection of relative expressions of CACNA2D1 and miR-107 in adjacent normal tissues and LSCC tissues (* P < 0.05). It was easily observed that they are inversely related to normal and LSCC tissues. LSCC, laryngeal squamous cell carcinoma; miR-107, microRNA-107; WB, Western blot.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 4. Differentiation properties of alpha 2 delta 1 + TU212 and TU686 cells. Flow cytometry shows the percentage of alpha 2 delta 1 + cells in TU212 and TU686 cells, FACS-purified alpha 2 delta 1 + cells and purified alpha 2 delta 1 + cells cultured in 10% serum-containing medium for 1 week (cultured).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry