Antibody data
- Antibody Data
- Antigen structure
- References [3]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [4]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 14-1579-37 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD157 Monoclonal Antibody (eBioSY11B5 (SY11B5)), eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: The eBioSY11B5 monoclonal antibody recognizes human CD157 (Mo5, BST-1). CD157 is a 42-45 kDa, GPI-anchored protein with structural and functional similarities with CD38. CD157 was initially cloned because of its expression on monocytes and macrophages, and was subsequently discovered to be the same protein named BST-1, discovered for its expression on bone marrow stromal cells and its ability to stimulate the proliferation of a mouse pre-B cell line. CD157 is a pleiotropic ectoenzyme and is thought to act independently as an enzyme and receptor. Similar to CD38, CD157 is involved in the metabolism of NAD+ and this activity may be involved in regulating intracellular Ca2+ levels. As a receptor, upon binding of its putative ligand, CD157 is thought to initiate a signal transduction cascade resulting in the phosphorylation of cytoplasmic proteins including focal adhesion kinase (FAK). The mechanism and functional significance of CD157-initiated signal transduction remain to be fully characterized. Applications Reported: This eBioSY11B5 (SY11B5) antibody has been reported for use in flow cytometric analysis, immunoprecipitation, immunoblotting (WB) (non-reduced only), and immunohistology staining of frozen tissue sections. Applications Tested: This eBioSY11B5 (SY11B5) antibody has been tested by flow cytometric analysis of normal human peripheral blood cells. This can be used at less than or equal to 0.5 µg per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. It is recommended that the antibody be carefully titrated for optimal performance in the assay of interest. Purity: Greater than 90%, as determined by SDS-PAGE. Aggregation: Less than 10%, as determined by HPLC. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- eBioSY11B5 (SY11B5)
- Vial size
- 2 mg
- Concentration
- 0.5 mg/mL
- Storage
- 4°C
Submitted references CD157 Confers Host Resistance to Mycobacterium tuberculosis via TLR2-CD157-PKCzeta-Induced Reactive Oxygen Species Production.
Enhancement of anti-leukemia activity of NK cells in vitro and in vivo by inhibition of leukemia cell-induced NK cell damage.
Novel SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic differentiation potential in mesenchymal stem cells.
Yang Q, Liao M, Wang W, Zhang M, Chen Q, Guo J, Peng B, Huang J, Liu H, Yahagi A, Xu X, Ishihara K, Cooper A, Chen X, Cai Y
mBio 2019 Aug 27;10(4)
mBio 2019 Aug 27;10(4)
Enhancement of anti-leukemia activity of NK cells in vitro and in vivo by inhibition of leukemia cell-induced NK cell damage.
Arriga R, Caratelli S, Coppola A, Spagnoli GC, Venditti A, Amadori S, Lanzilli G, Lauro D, Palomba P, Sconocchia T, Del Principe MI, Maurillo L, Buccisano F, Capuani B, Ferrone S, Sconocchia G
Oncotarget 2016 Jan 12;7(2):2070-9
Oncotarget 2016 Jan 12;7(2):2070-9
Novel SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic differentiation potential in mesenchymal stem cells.
Aomatsu E, Takahashi N, Sawada S, Okubo N, Hasegawa T, Taira M, Miura H, Ishisaki A, Chosa N
Scientific reports 2014 Jan 13;4:3652
Scientific reports 2014 Jan 13;4:3652
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
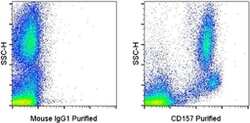
- Experimental details
- Staining of normal human peripheral blood cells with 0.25 µg of Mouse IgG1 K Isotype Control Purified (Product # 14-4714-82) (left) or 0.25 µg of Anti-Human CD157 Purified (right) followed by F (ab')2 Anti-Mouse IgG PE (Product # 12-4010-82).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
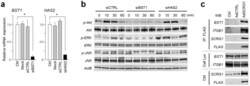
- Experimental details
- Figure 3 SCRG1 receptor BST1 relays ERK and PI3K signals in hMSCs. (a) UE7T13 cells were transfected with siRNA for BST1 (siBST1) or HAS2 (siHAS2). mRNA expression of BST1 (left panel) and HAS2 (right panel) was measured by qRT-PCR and normalized to GAPDH; results are expressed as fold increase or decrease relative to the control (Ctrl). Data are presented as mean +- SD. * p < 0.05 was considered significant. (b) UE7T-13 cells were transfected with siBST1 or siHAS2. After transfection for 48 h, the cells were serum-starved overnight and then stimulated with 500 ng/mL rhSCRG1. The cells were washed twice with ice-cold PBS and lysed in RIPA buffer. Samples were separated by SDS-PAGE and analyzed by western blotting. Although cropped blots were used, the gels were run under the same experimental conditions. (c) UE7T-13 cells were transfected with pAdSCRG1-FLAG. After 1 week, the cells were treated with 0.5 mM dithiobis-sulfosuccinimidylpropionate and then lysed in ice-cold RIPA buffer. Lysates were incubated with anti-FLAG M2 Agarose Affinity Gel and proteins were eluted by adding Laemmli sample buffer (IP: FLAG). Eluted proteins were analyzed by western blotting. Total cell lysate (Cell Lys) and 5-fold concentrated conditioned medium (CM) were used as loading controls. Although cropped blots were used, the gels were run under the same experimental conditions.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
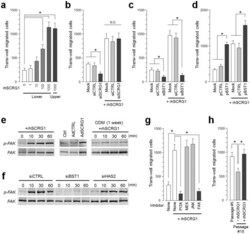
- Experimental details
- Figure 4 SCRG1/BST1 stimulates FAK/PI3K-dependent hMSC migration and preserves migratory activity after ex vivo expansion. (a) Migration of UE7T-13 cells was investigated as described in the Methods. rhSCRG1 was added at various concentrations (1-1000 ng/mL). After incubation for 15 h, the number of cells that had migrated to the lower side was counted. (b and c) UE7T-13 cells were transfected with siSCRG1 (b) or siBST1 (c). rhSCRG1 (500 ng/mL) was added to the lower well of the Transwell plate and trans-well migration was analyzed as described in (a). (d) UE7T-13 cells were transfected with pCMV--IRES-AcGFP (pCTRL) or pCMV-BST1-IRES-AcGFP (pBST1). rhSCRG1 (500 ng/mL) was added to the lower well of the Transwell plate and trans-well migration was analyzed as described in (a). (e) Phosphorylation of FAK was detected in UE7T-13 as in Figs. 2a-c . Although cropped blots were used, the gels were run under the same experimental conditions. (f) Phosphorylation of FAK was detected in UE7T-13 as in Fig. 3b . Although cropped blots were used, the gels were run under the same experimental conditions. (g) UE7T-13 cells were treated with 1 muM kinase inhibitors trans-well migration was analyzed as in (a). PI3K inhibitor LY294002 (PI3K), MEK inhibitor U0126 (MEK), JNK inhibitor SP600125 (JNK), and FAK inhibitor I (FAK) were added to the lower and upper wells. (h) Primary cultured hMSCs (passage number #5) were subcultured ten times in the presence (passage number #15, +rhSCRG1) or abs
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
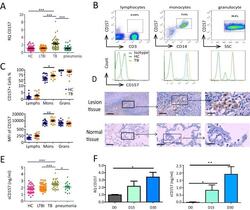
- Experimental details
- FIG 1 CD157 expression levels are significantly increased in patients with TB. (A) Cd157 expression levels in whole blood from HC subjects ( n = 55), LTBI subjects ( n = 46), TB patients ( n = 51), and pneumonia patients ( n = 38) were determined by quantitative PCR (qPCR). RQ, relative quantification. (B) Peripheral blood was stained with anti-CD3, anti-CD14, and anti-CD157 antibodies and analyzed by flow cytometry. The histograms show the percentage of CD157-positive cells in lymphocytes, monocytes, and granulocytes from peripheral blood. SSC, side scatter. (C) The percentage (top panel) and mean fluorescence intensity (MFI) (bottom panel) of CD157 expression on lymphocytes (Lymphs), monocytes (Mons), and granulocytes (Grans) from HC subjects ( n = 21) and TB patients ( n = 20). (D) Immunohistochemical analysis of CD157 expression in tuberculous granuloma of lung tissue samples from patients with active tuberculosis (top panels) and in normal lung tissue adjacent to the lesions from patients with TB (bottom panels). Brown-labeled cells (arrow) represent CD157-positive cells. The images of the whole microscope slides were captured using a NanoZoomer digital pathology system (Hamamatsu Photonics). Outlined areas in the main images are enlarged in the insets. The bars represent 100 mum. Images that were representative of the images from three experiments were presented. (E) The concentrations of sCD157 in plasma from HC subjects ( n = 46), LTBI subjects ( n = 46), TB patients
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
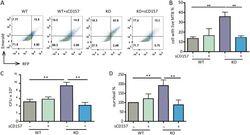
- FIG 3 CD157 deficiency impairs macrophage bactericidal capacity. Peritoneal macrophages from WT mice and Cd157 KO mice treated with sCD157 (+sCD157) (5 mug/ml) or not treated with sCD157 were infected with strain H37Ra harboring a dual-color reporter that comprises a constitutively green (Emerald) and a tetracycline-inducible red (TagRFP) fluorescent protein for 3 days. Tetracycline (500 ng/ml) was added 24 h before flow cytometry. Macrophages were then harvested and fixed with 4% PFA, and the percentage of cells with live M. tuberculosis (MTB) (red) were determined by FACS. The data are presented as representative dot plot data (A) and means plus SEM (B to D). Bacterial burden within peritoneal macrophages from WT mice and Cd157 KO mice at 72 h postinfection with the H37Rv strain (MOI of 5) in the presence or absence of sCD157 (5 mug/ml). The data were presented as absolute number of CFU (C) or the percentage of survival relative to WT mock treatment (D). One-way ANOVA Newman-Keuls multiple comparison test (B, C, and D) was used. * * , P
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Flow cytometry
Flow cytometry