Antibody data
- Antibody Data
- Antigen structure
- References [11]
- Comments [0]
- Validations
- Immunocytochemistry [5]
- Immunoprecipitation [1]
- Immunohistochemistry [2]
- Chromatin Immunoprecipitation [4]
- Other assay [9]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-27157 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- FOXA1 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- Recommended positive controls: A431, HepG2. Predicted reactivity: Mouse (100%), Rat (100%), Zebrafish (100%), Xenopus laevis (100%), Bovine (100%). Store product as a concentrated solution. Centrifuge briefly prior to opening the vial.
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 0.44 mg/mL
- Storage
- Store at 4°C short term. For long term storage, store at -20°C, avoiding freeze/thaw cycles.
Submitted references Targeting SWI/SNF ATPases in enhancer-addicted prostate cancer.
Comprehensive Gene Expression Analyses of Immunohistochemically Defined Subgroups of Muscle-Invasive Urinary Bladder Urothelial Carcinoma.
STK11/LKB1 Loss of Function Is Associated with Global DNA Hypomethylation and S-Adenosyl-Methionine Depletion in Human Lung Adenocarcinoma.
PD-L1 Expression in Muscle-Invasive Urinary Bladder Urothelial Carcinoma According to Basal/Squamous-Like Phenotype.
Non-Muscle-Invasive Bladder Carcinoma with Respect to Basal Versus Luminal Keratin Expression.
Aberrant activation of CYR61 enhancers in colorectal cancer development.
Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer.
Transcriptional Analysis of Immunohistochemically Defined Subgroups of Non-Muscle-Invasive Papillary High-Grade Upper Tract Urothelial Carcinoma.
Androgen Receptor-Activated Enhancers Simultaneously Regulate Oncogene TMPRSS2 and lncRNA PRCAT38 in Prostate Cancer.
Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression.
FOXA1 knock-out via CRISPR/Cas9 altered Casp-9, Bax, CCND1, CDK4, and fibronectin expressions in LNCaP cells.
Xiao L, Parolia A, Qiao Y, Bawa P, Eyunni S, Mannan R, Carson SE, Chang Y, Wang X, Zhang Y, Vo JN, Kregel S, Simko SA, Delekta AD, Jaber M, Zheng H, Apel IJ, McMurry L, Su F, Wang R, Zelenka-Wang S, Sasmal S, Khare L, Mukherjee S, Abbineni C, Aithal K, Bhakta MS, Ghurye J, Cao X, Navone NM, Nesvizhskii AI, Mehra R, Vaishampayan U, Blanchette M, Wang Y, Samajdar S, Ramachandra M, Chinnaiyan AM
Nature 2022 Jan;601(7893):434-439
Nature 2022 Jan;601(7893):434-439
Comprehensive Gene Expression Analyses of Immunohistochemically Defined Subgroups of Muscle-Invasive Urinary Bladder Urothelial Carcinoma.
Kim B, Jang I, Kim K, Jung M, Lee C, Park JH, Kim YA, Moon KC
International journal of molecular sciences 2021 Jan 10;22(2)
International journal of molecular sciences 2021 Jan 10;22(2)
STK11/LKB1 Loss of Function Is Associated with Global DNA Hypomethylation and S-Adenosyl-Methionine Depletion in Human Lung Adenocarcinoma.
Koenig MJ, Agana BA, Kaufman JM, Sharpnack MF, Wang WZ, Weigel C, Navarro FCP, Amann JM, Cacciato N, Arasada RR, Gerstein MB, Wysocki VH, Oakes C, Carbone DP
Cancer research 2021 Aug 15;81(16):4194-4204
Cancer research 2021 Aug 15;81(16):4194-4204
PD-L1 Expression in Muscle-Invasive Urinary Bladder Urothelial Carcinoma According to Basal/Squamous-Like Phenotype.
Kim B, Lee C, Kim YA, Moon KC
Frontiers in oncology 2020;10:527385
Frontiers in oncology 2020;10:527385
Non-Muscle-Invasive Bladder Carcinoma with Respect to Basal Versus Luminal Keratin Expression.
Jung M, Jang I, Kim K, Moon KC
International journal of molecular sciences 2020 Oct 19;21(20)
International journal of molecular sciences 2020 Oct 19;21(20)
Aberrant activation of CYR61 enhancers in colorectal cancer development.
Xie L, Song X, Lin H, Chen Z, Li Q, Guo T, Xu T, Su T, Xu M, Chang X, Wang LK, Liang B, Huang D
Journal of experimental & clinical cancer research : CR 2019 May 22;38(1):213
Journal of experimental & clinical cancer research : CR 2019 May 22;38(1):213
Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer.
Parolia A, Cieslik M, Chu SC, Xiao L, Ouchi T, Zhang Y, Wang X, Vats P, Cao X, Pitchiaya S, Su F, Wang R, Feng FY, Wu YM, Lonigro RJ, Robinson DR, Chinnaiyan AM
Nature 2019 Jul;571(7765):413-418
Nature 2019 Jul;571(7765):413-418
Transcriptional Analysis of Immunohistochemically Defined Subgroups of Non-Muscle-Invasive Papillary High-Grade Upper Tract Urothelial Carcinoma.
Jung M, Lee JH, Kim B, Park JH, Moon KC
International journal of molecular sciences 2019 Jan 29;20(3)
International journal of molecular sciences 2019 Jan 29;20(3)
Androgen Receptor-Activated Enhancers Simultaneously Regulate Oncogene TMPRSS2 and lncRNA PRCAT38 in Prostate Cancer.
Chen Z, Song X, Li Q, Xie L, Guo T, Su T, Tang C, Chang X, Liang B, Huang D
Cells 2019 Aug 9;8(8)
Cells 2019 Aug 9;8(8)
Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression.
Zhang Y, Pitchiaya S, Cieślik M, Niknafs YS, Tien JC, Hosono Y, Iyer MK, Yazdani S, Subramaniam S, Shukla SK, Jiang X, Wang L, Liu TY, Uhl M, Gawronski AR, Qiao Y, Xiao L, Dhanasekaran SM, Juckette KM, Kunju LP, Cao X, Patel U, Batish M, Shukla GC, Paulsen MT, Ljungman M, Jiang H, Mehra R, Backofen R, Sahinalp CS, Freier SM, Watt AT, Guo S, Wei JT, Feng FY, Malik R, Chinnaiyan AM
Nature genetics 2018 Jun;50(6):814-824
Nature genetics 2018 Jun;50(6):814-824
FOXA1 knock-out via CRISPR/Cas9 altered Casp-9, Bax, CCND1, CDK4, and fibronectin expressions in LNCaP cells.
Albayrak G, Konac E, Ugras Dikmen A, Bilen CY
Experimental biology and medicine (Maywood, N.J.) 2018 Aug;243(12):990-994
Experimental biology and medicine (Maywood, N.J.) 2018 Aug;243(12):990-994
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
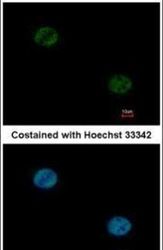
- Experimental details
- Immunofluorescent analysis of FOXA1 in paraformaldehyde-fixed HeLa cells using a FOXA1 polyclonal antibody (Product # PA5-27157) at a 1:1000 dilution.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
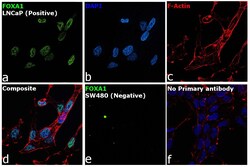
- Experimental details
- Immunofluorescence analysis of FOXA1 was performed using 70% confluent log phase LNCaP and SW480 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with FOXA1 Polyclonal Antibody (Product # PA5-27157) at 1:200 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Recombinant Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034), (1:3000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained withRhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Nuclear localization. Panel e represents image for SW480 cells showing no staining for FOXA1. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
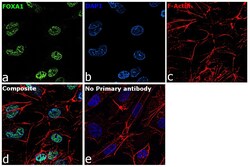
- Experimental details
- Immunofluorescence analysis of FOXA1 was performed using 70% confluent log phase Hep G2 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with FOXA1 Polyclonal Antibody (Product # PA5-27157) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
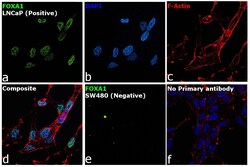
- Experimental details
- Immunofluorescence analysis of FOXA1 was performed using 70% confluent log phase LNCaP and SW480 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with FOXA1 Polyclonal Antibody (Product # PA5-27157) at 1:200 dilution in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Recombinant Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034), (1:3000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b: Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained withRhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Nuclear localization. Panel e represents image for SW480 cells showing no staining for FOXA1. Panel f represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
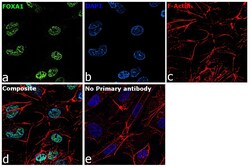
- Experimental details
- Immunofluorescence analysis of FOXA1 was performed using 70% confluent log phase Hep G2 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with FOXA1 Polyclonal Antibody (Product # PA5-27157) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32731), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Nuclear localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
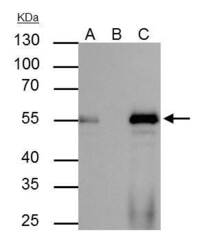
- Experimental details
- FOXA1 Polyclonal Antibody immunoprecipitates FOXA1 protein in IP experiments. IP Sample: HepG2 whole cell lysate/extract A : 40 µg whole cell lysate/extract of FOXA1 protein expressing HepG2 cells B : Control with 2.5 µg of pre-immune rabbit IgG C : Immunoprecipitation of FOXA1 by 2.5 µg of FOXA1 Polyclonal Antibody (Product # PA5-27157) 10% SDS-PAGE The immunoprecipitated FOXA1 protein was detected by FOXA1 Polyclonal Antibody (Product # PA5-27157) diluted at 1:1,000. Anti-rabbit IgG (HRP) was used as a secondary reagent.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
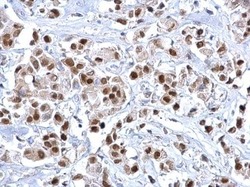
- Experimental details
- FOXA1 Polyclonal Antibody detects FOXA1 protein at nucleus on human breast carcinoma by immunohistochemical analysis. Sample: Paraffin-embedded human breast carcinoma. FOXA1 Polyclonal Antibody (Product # PA5-27157) dilution: 1:500. Antigen Retrieval: EDTA based buffer, pH 8.0, 15 min.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
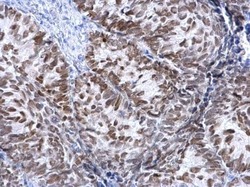
- Experimental details
- FOXA1 Polyclonal Antibody detects FOXA1 protein at nucleus on human cervical carcinoma by immunohistochemical analysis. Sample: Paraffin-embedded human cervical carcinoma. FOXA1 Polyclonal Antibody (Product # PA5-27157) dilution: 1:500. Antigen Retrieval: EDTA based buffer, pH 8.0, 15 min.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
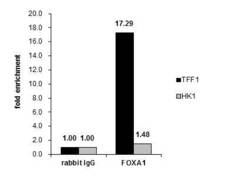
- Experimental details
- Cross-linked ChIP was performed with MCF-7 chromatin extract and 5 µg of either control rabbit IgG or FOXA1 Polyclonal Antibody (Product # PA5-27157). The precipitated DNA was detected by PCR with primer set targeting to TFF1 or HK1.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
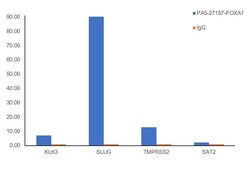
- Experimental details
- Chromatin Immunoprecipitation (ChIP) assay of endogenous FOXA1 protein using FOXA1 Antibody: ChIP was performed using Anti-FOXA1 Polyclonal Antibody (Product # PA5-27157, 2.5 µg) on sheared chromatin from LnCaP cells using the MAGnify ChIP System kit (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA was analyzed by qPCR using primers binding to KLK3, SLµg, TMPRSS2 (Active) and SAT2 satellite repeats (Inactive). Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
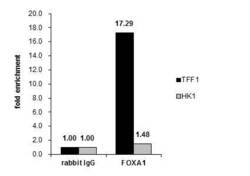
- Experimental details
- Cross-linked ChIP was performed with MCF-7 chromatin extract and 5 µg of either control rabbit IgG or FOXA1 Polyclonal Antibody (Product # PA5-27157). The precipitated DNA was detected by PCR with primer set targeting to TFF1 or HK1.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
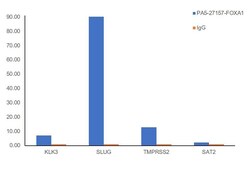
- Experimental details
- Chromatin Immunoprecipitation (ChIP) assay of endogenous FOXA1 protein using FOXA1 Antibody: ChIP was performed using Anti-FOXA1 Polyclonal Antibody (Product # PA5-27157, 2.5 µg) on sheared chromatin from LnCaP cells using the MAGnify ChIP System kit (Product # 49-2024). Normal Rabbit IgG was used as a negative IP control. The purified DNA was analyzed by qPCR using primers binding to KLK3, SLµg, TMPRSS2 (Active) and SAT2 satellite repeats (Inactive). Data is presented as fold enrichment of the antibody signal versus the negative control IgG using the comparative CT method.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
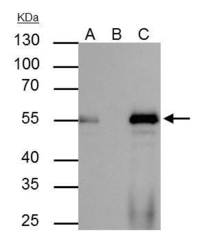
- FOXA1 Polyclonal Antibody immunoprecipitates FOXA1 protein in IP experiments. IP Sample: HepG2 whole cell lysate/extract A : 40 µg whole cell lysate/extract of FOXA1 protein expressing HepG2 cells B : Control with 2.5 µg of pre-immune rabbit IgG C : Immunoprecipitation of FOXA1 by 2.5 µg of FOXA1 Polyclonal Antibody (Product # PA5-27157) 10% SDS-PAGE The immunoprecipitated FOXA1 protein was detected by FOXA1 Polyclonal Antibody (Product # PA5-27157) diluted at 1:1,000. Anti-rabbit IgG (HRP) was used as a secondary reagent.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
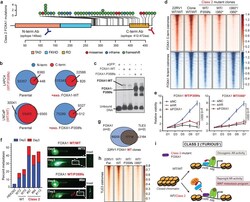
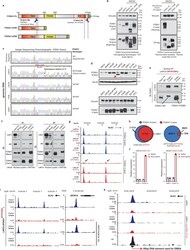
- Figure 3 Functional characterization of Class2 mutations of FOXA1. a) Class2 mutations and antibody epitopes on the protein map of FOXA1. b) N-term and C-term FOXA1 cistromes in PCa cells that are ( right ) untreated or (left) have exogenous overexpression of FOXA1 variants. c) Electromobility shift of FOXA1 variants bound to the KLK3-enhancer (n=3 biological replicates). For gel source data, see Supplementary Figure 1 . d) FOXA1 ChIP-seq read-density heatmaps in independent class2-mutant 22RV1 CRISPR clones. e) Growth of class2-mutant 22RV1 clones treated with non-targeting (siNC), AR or FOXA1 targeting siRNAs (n=5 biological replicates; two-way ANOVA and Tukey's test). Mean +- s.e.m. are shown. f) Left, Metastasis frequency in zebrafish embryos injected with HEK293 (negative control), WT, or class2-mutant 22RV1 clones (n>=30 embryos/group); Right, representative embryo images showing the disseminated PCa cells. g) Overlap of WT FOXA1 and TLE3 binding sites in 22RV1 CRISPR clones (n=2 biological replicates each). h) TLE3 ChIP-seq read-density heatmaps in two distinct FOXA1 WT and class2-mutant 22RV1 clones. i) Class2 model: Truncated FOXA1 shows dominant chromatin binding and displaces WT FOXA1 and TLE3 from the chromatin, resulting in increased WNT-signaling. FKRE, forkhead responsive element; ARE, androgen responsive element.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
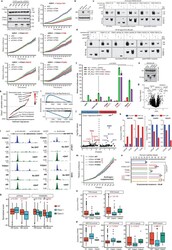
- Extended Data Figure 4 Functional impact of FOXA1 mutations on oncogenic AR-signaling. a) Immunoblot showing expression of endogenous and V5-tagged exogenous FOXA1 proteins in dox-inducible 22RV1 cells transfected with distinct UTR-specific FOXA1 siRNAs (#3-5) or a non-targeting control siRNA (siNC). These results represent 2 independent experiments. Incucyte growth curves of 22RV1 cells overexpressing empty vector (control), WT, or mutant FOXA1 variants upon treatment with UTR-specific FOXA1-targeting siRNAs (n = 5 biological replicates). Mean +- s.e.m are shown. b) Immunoblots confirming stable overexpression of the WT AR protein in HEK293 and PC3 cells. c, d) Co-immunoprecipitation assay of indicated recombinant FOXA1 variants using a V5-tag antibody in (c) HEK293-AR and (d) PC3-AR cells. eGFP is a negative control; FL is the full-length WT FOXA1. d168 and d358 are truncated FOXA1 variants with only the first 168aa (i.e. before the Forkhead domain) or 358aa of FOXA1 protein. H247Q and R261G are missense class1 mutant variants. e) Immunoblots confirming comparable expression of AR and recombinant FOXA1 variants in AR reporter assay-matched HEK293 lysates. Immunoblots show representative results from 2-3 independent experiments and class1 and class2 mutants serve as biological replicates. For all gel source data ( a,b-e ), see Supplementary Figure 1 . f) AR dual-luciferase reporter assays with transient overexpression of indicated FOXA1 variant in HEK293-AR cells with or wit
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Extended Data Figure 5 DNA-binding dominance of the Class2 FOXA1 mutants. a) FOXA1 protein maps showing the recombinant proteins used to validate the N-terminal (N-term) and C-terminal (C-term) FOXA1 antibodies. TAD, trans-activating domain; FKDH, Forkhead domain; RD, regulatory domain. b) Immunoblots depicting detection of all variants by the N-term antibody (left), and of only the full-length WT FOXA1 protein by the C-term antibody (right). These results were reproducible in 2 independent experiments. Antibody details are included in the Methods. c) Sanger sequencing chromatograms showing the heterozygous class2 mutation in LAPC4 cells after the P358 codon in Exon2 (n=2 technical replicates). All other tested PCa cell lines were WT for FOXA1. d) Immunoblots confirming the expression of the truncated FOXA1 variant in LAPC4 at the expected ~40kDa size (top, red arrow). The short band is detectable only with the N-term (top) FOXA1 antibody and not the C-term (bottom) antibody. These results were reproducible in 2 independent experiments. e) Co-immunoprecipitation and immunoblotting of FOXA1 using a N-term and C-term antibodies from LAPC4 nuclei with species-matched IgG used as control. f) Nuclear co-immunoprecipitation of FOXA1 from LAPC4 or LNCaP cells stimulated with DHT (10nM for 16h) using N-term and C-term antibodies. Species-matched IgG are controls. Immunoprecipitations and immunoblots in d-f were reproducible in 2 and 3 independent experiments, respectively. For gel so
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
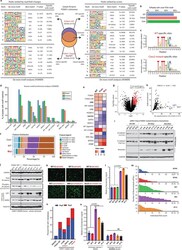
- Extended Data Figure 7 Cistromic and WNT-driven phenotypic characteristics of the Class2 FOXA1 mutants. a) De novo motif analyses of the WT-specific, common, and class2-specific FOXA1 binding site subsets defined from either (left) sequencing read fold-changes or (right) peak-calling scores of ChIP-seq data in 7a . WT and class2 cistromes were generated from n=3 and n=2 independent biological replicates, respectively. Only the top 5K or 10K peaks from each subset were used as inputs for motif discovery (see Methods ; HOMER: hypergeometric test). b) Percent of WT or class2 binding sites with perfect match to the core FOXA1 motif (5'-T[G/A]TT[T/G]AC-3') and c) the consensus FOXA1 motifs identified from these sites. d) Percent of binding sites in the three FOXA1 binding site subsets harboring known motifs of the labelled FOXA1 or AR cofactors, and e) enrichment of the cofactor motifs in the three binding site subsets relative to the background. f) Genomic distribution of WT-specific, common and class2-specific binding sites in PCa cells. g) Differential expression of genes in FOXA1 class2 mutant CRISPR clones relative to FOXA1 WT clones (n=2 biological replicates (Limma two-sided test). h) Distinct TF motifs within the promoter (2kb upstream) of differentially expressed genes. TFs with the highest enrichment (fold-change, percent of up-regulated genes with the motif, and significance) are highlighted and labeled (two-tailed Fisher's exact test). i) Immunoblots showing the expres
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
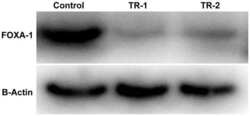
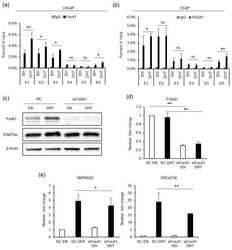
- Figure 5 FOXA1 is recruited by the AR to the enhancers and regulates the transcription of TMPRSS2 and PRCAT38 . ( a , b ) ChIP-qPCR before and after DHT treatment showing FOXA1 enrichment over the enhancer regions in LNCaP and VCaP. ( c ) Western blot showing the protein level of FOXA1 and H3K27ac after FOXA1 knockdown with or without DHT treatment. ( d - e ) qPCR showing the down-regulation of FOXA1 , TMPRSS2 and PRCAT38 after FOXA1 knockdown with or without DHT treatment. Data are shown as mean +- SD (n = 3).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
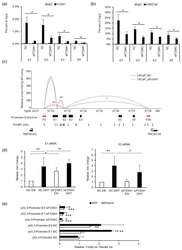
- Figure 6 FOXA1 is required for enhancer activity and chromatin looping between enhancers and promoters of TMPRSS2 and PRCAT38 . ( a , b ) ChIP-qPCR in LNCaP cells before and after FOXA1 knockdown showing FOXA1 and H3K27ac enrichment over the enhancer regions. ( c ) 3C-qPCR analysis of the looping between enhancers and promoters before and after FOXA1 knockdown in LNCaP (peak values of the loops are the mean of 3 biological replicates). ( d ) eRNA transcription from enhancers E1 and E2 before and after FOXA1 knockdown in LNCaP. ( e ) Dual-luciferase reporter assay to determine enhancer activity of E1 and E2 before and after FOXA1 knockdown in LNCaP. Data are shown as the mean +- SD (n = 3); * indicates a significant difference between vehicle and DHT treatment, + indicates significant difference between respective construct and pGL3-promoter. */+: P < 0.05, **/++: P < 0.01, ns: not significant.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
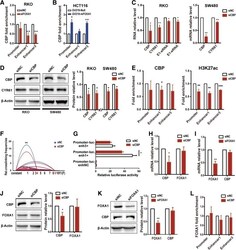
- Experimental details
- Fig. 4 CBP is recruited by FOXA1 to up-regulate CYR61 enhancer activity and CYR61 expression. a CBP enrichment decreased at Enhancer1 and Enhancer3 after treatment with FOXA1 siRNA for 72 h in RKO cells, and b increased at the promoter and Enhancer3 after FOXA1 over-expression for 48 h in HCT116 cells, as assessed by ChIP-qPCR. c CYR61 mRNA and eRNA, and d protein levels were decreased after treatment with CBP siRNA for 26 h in RKO and SW480 cell lines. e CBP and H3K27ac enrichment was decreased after treatment with CBP siRNA for 26 h in RKO cells, as assessed by ChIP-qPCR. f Relative crosslinking frequencies of CYR61 promoter/Enhancer1 were decreased after treatment with CBP siRNA for 26 h in RKO cells, as assessed by 3C; significance determined by the paired-samples t-test. g Relative activity of promoter and enhancers after treatment with CBP siRNA for 26 h in RKO cells, as assessed by relative luciferase reporter gene expression. CBP and FOXA1 mRNA expression levels in RKO cells after treatment with CBP siRNA for 26 h h or with FOXA1 siRNA for 72 h i . CBP and FOXA1 protein expression in RKO cells after treatment with CBP siRNA for 26 h j or with FOXA1 siRNA for 72 h k . (L) FOXA1 enrichment after treatment with CBP siRNA for 26 h in RKO cells, as assessed by ChIP-qPCR. Significance for all data except f was determined by the independent samples t-test. Data are shown as mean +- S.D., n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry