Antibody data
- Antibody Data
- Antigen structure
- References [12]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Flow cytometry [1]
- Other assay [5]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-904 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- IP3 Receptor 2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-904 detects inositol 1,4,5-trisphosphate receptor type-II (IP3R-II) from rat and mouse tissues. PA1-904 has been successfully used in immunohistochemistry, immunofluorescence and immunocytochemistry procedures. Immunohistochemical staining of IP3R-II in rat brain with PA1-904 results in staining of the hippocampus, corpus collosum, and cerebellum. This product fails to detect IP3R-II in Western blot procedures. PA1-904 immunizing peptide corresponds to amino acid residues 317-334 from rat IP3R-II. PA1-904 immunizing peptide (Cat. # PEP-024) is available for use in neutralization and control experiments.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Paclitaxel accelerates spontaneous calcium oscillations in cardiomyocytes by interacting with NCS-1 and the InsP3R.
Glycosaminoglycan mimetics trigger IP3-dependent intracellular calcium release in myoblasts.
Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry.
Mini-dystrophin expression down-regulates overactivation of G protein-mediated IP3 signaling pathway in dystrophin-deficient muscle cells.
Mini-dystrophin expression down-regulates IP3-mediated calcium release events in resting dystrophin-deficient muscle cells.
Immunolocalization of type 2 inositol 1,4,5-trisphosphate receptors in cardiac myocytes from newborn mice.
Expression of calcium transporters in the retina of the tiger salamander (Ambystoma tigrinum).
C(a2+)-dependent glutamate release involves two classes of endoplasmic reticulum Ca(2+) stores in astrocytes.
ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors.
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Sparks and puffs in oligodendrocyte progenitors: cross talk between ryanodine receptors and inositol trisphosphate receptors.
Zhang K, Heidrich FM, DeGray B, Boehmerle W, Ehrlich BE
Journal of molecular and cellular cardiology 2010 Nov;49(5):829-35
Journal of molecular and cellular cardiology 2010 Nov;49(5):829-35
Glycosaminoglycan mimetics trigger IP3-dependent intracellular calcium release in myoblasts.
Martelly I, Singabraya D, Vandebrouck A, Papy-Garcia D, Cognard C, Raymond G, Guillet-Deniau I, Courty J, Constantin B
Matrix biology : journal of the International Society for Matrix Biology 2010 May;29(4):317-29
Matrix biology : journal of the International Society for Matrix Biology 2010 May;29(4):317-29
Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry.
Cheshenko N, Liu W, Satlin LM, Herold BC
Molecular biology of the cell 2007 Aug;18(8):3119-30
Molecular biology of the cell 2007 Aug;18(8):3119-30
Mini-dystrophin expression down-regulates overactivation of G protein-mediated IP3 signaling pathway in dystrophin-deficient muscle cells.
Balghi H, Sebille S, Constantin B, Patri S, Thoreau V, Mondin L, Mok E, Kitzis A, Raymond G, Cognard C
The Journal of general physiology 2006 Feb;127(2):171-82
The Journal of general physiology 2006 Feb;127(2):171-82
Mini-dystrophin expression down-regulates IP3-mediated calcium release events in resting dystrophin-deficient muscle cells.
Balghi H, Sebille S, Mondin L, Cantereau A, Constantin B, Raymond G, Cognard C
The Journal of general physiology 2006 Aug;128(2):219-30
The Journal of general physiology 2006 Aug;128(2):219-30
Immunolocalization of type 2 inositol 1,4,5-trisphosphate receptors in cardiac myocytes from newborn mice.
García KD, Shah T, García J
American journal of physiology. Cell physiology 2004 Oct;287(4):C1048-57
American journal of physiology. Cell physiology 2004 Oct;287(4):C1048-57
Expression of calcium transporters in the retina of the tiger salamander (Ambystoma tigrinum).
Krizaj D, Liu X, Copenhagen DR
The Journal of comparative neurology 2004 Aug 2;475(4):463-80
The Journal of comparative neurology 2004 Aug 2;475(4):463-80
C(a2+)-dependent glutamate release involves two classes of endoplasmic reticulum Ca(2+) stores in astrocytes.
Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V
Journal of neuroscience research 2004 Apr 1;76(1):86-97
Journal of neuroscience research 2004 Apr 1;76(1):86-97
ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors.
Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS
American journal of physiology. Lung cellular and molecular physiology 2003 Sep;285(3):L680-90
American journal of physiology. Lung cellular and molecular physiology 2003 Sep;285(3):L680-90
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC
BMC neuroscience 2001;2:6
BMC neuroscience 2001;2:6
Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction.
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC
BMC neuroscience 2001;2:6
BMC neuroscience 2001;2:6
Sparks and puffs in oligodendrocyte progenitors: cross talk between ryanodine receptors and inositol trisphosphate receptors.
Haak LL, Song LS, Molinski TF, Pessah IN, Cheng H, Russell JT
The Journal of neuroscience : the official journal of the Society for Neuroscience 2001 Jun 1;21(11):3860-70
The Journal of neuroscience : the official journal of the Society for Neuroscience 2001 Jun 1;21(11):3860-70
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
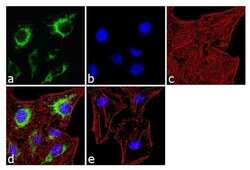
- Experimental details
- Immunofluorescence analysis of IP3 Receptor II was done on 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with IP3 Receptor II Rabbit Polyclonal Antibody (Product # PA1-904) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
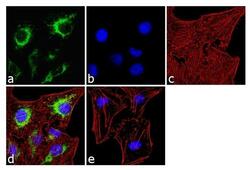
- Experimental details
- Immunofluorescence analysis of IP3 Receptor II was done on 70% confluent log phase SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with IP3 Receptor II Rabbit Polyclonal Antibody (Product # PA1-904) at 2 µg/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (Heavy Chain) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (Product # S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
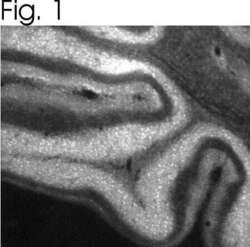
- Experimental details
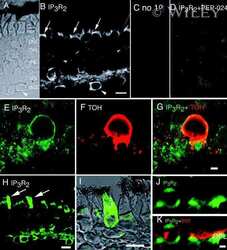
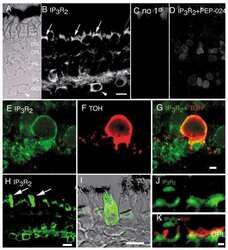
- Immunolocalization of IP3R-II in rat brain using Product # PA1-904.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
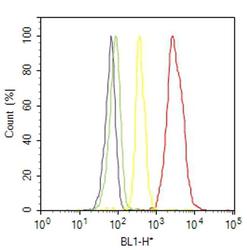
- Experimental details
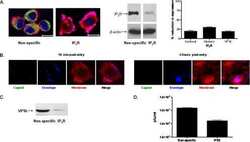
- Flow cytometry analysis of IP3 Receptor II was done on SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with IP3 Receptor II Rabbit Polyclonal Antibody (PA1904, red histogram) or with rabbit isotype control (yellow histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
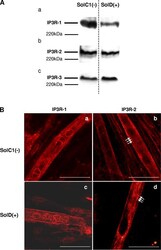
- Figure 5. Determination and immunolocalization of the expression pattern of IP3R proteins. (A) Western blot analysis of IP3R-1 (a), IP3R-2 (b), and IP3R-3 (c), respectively, in SolC1(-) and SolD(+) myotubes. 30 mug protein from each type of myotube was separated on 5% SDS-PAGE, transferred onto a pore nitrocellulose membrane, and processed for Western blot analysis with specific antibodies. The 220-kD band corresponded to the molecular weight of the myosin protein. (B) IP3R isoform immunolabeling in SolC1(-) and SolD(+) myotubes was observed with laser scanning confocal microscopy. IP3R-1 and IP3R-2 localizations were detected using polyclonal antibodies, PA1-901 and PA1-904, respectively. IP3R-1 staining is localized in the nuclear envelope region and in the cytoplasm in SolC1(-) (a) and in SolD(+) (c) myotubes. IP3R-2 showed a peripheral and fully cytoplasmic localization and, as indicated with white arrows, striated staining pattern appeared at this differentiation stage in SolC1(-) (b) and SolD(+) (d) myotubes. Bars, 25 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
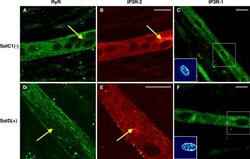
- Figure 7. Immunolocalization of RyRs and IP3Rs in SolC1(-) and SolD(+) myotubes. Immunolabelings of SR calcium release channels in SolC1(-) (A-C) and in SolD(+) myotubes (D-F) were observed with laser scanning confocal microscopy (LSCM). The same random distribution of RyRs (anti-RyR; Calbiochem) was observed in SolC1(-) myotubes (A) and in SolD(+) myotubes (D). In the same myotubes, immunostaining with antibodies against IP3R-2 was performed in SolC1(-) (B) and in SolD(+) (E). Yellow arrows, locations corresponding to elevated staining for IP3R-2 and low staining for RyR. IP3R-1 staining is localized in the nuclear envelope region and in the cytoplasm in SolC1(-) (C) and in SolD(+) (F) myotubes. Insets, staining of selected nuclei (dotted squares) loaded with the fluorescent TO-PRO-3 probe. IP3R-1 and IP3R-2 localizations were detected using polyclonal antibodies, PA1-901 and PA1-904, respectively.
 Explore
Explore Validate
Validate Learn
Learn Immunocytochemistry
Immunocytochemistry