12-3109-41
antibody from Invitrogen Antibodies
Targeting: HAVCR2
CD366, FLJ14428, Tim-3, TIM3, TIMD3
Antibody data
- Antibody Data
- Antigen structure
- References [15]
- Comments [0]
- Validations
- Flow cytometry [2]
- Other assay [2]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 12-3109-41 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CD366 (TIM3) Monoclonal Antibody (F38-2E2), PE, eBioscience™
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- Description: This F38-2E2 monoclonal antibody reacts with human CD366, also known as T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) or HAVCR2. This cell surface receptor is expressed on activated CD4+ T cell subsets (e.g. Th1, Th17, and Treg), CD8+ T cells, monocytes, dendritic cells, and mast cells. Due to alternative splicing, CD366 exists as membrane-bound and soluble forms. Galectin-9 has been identified as the ligand for CD366. In humans, this receptor negatively regulates CD4+ T cells, influencing the secretion of some Th1- and Th17-related cytokines. CD366 has also been implicated in tolerance, autoimmune disease (e.g. multiple sclerosis), and HIV infection. Applications Reported: This F38-2E2 antibody has been reported for use in flow cytometric analysis. Applications Tested: This F38-2E2 antibody has been pre-titrated and tested by flow cytometric analysis of stimulated normal human peripheral blood cells. This can be used at 5 µL (0.125 µg) per test. A test is defined as the amount (µg) of antibody that will stain a cell sample in a final volume of 100 µL. Cell number should be determined empirically but can range from 10^5 to 10^8 cells/test. Excitation: 488-561 nm; Emission: 578 nm; Laser: Blue Laser, Green Laser, Yellow-Green Laser. Filtration: 0.2 µm post-manufacturing filtered.
- Reactivity
- Human
- Host
- Mouse
- Conjugate
- Yellow dye
- Isotype
- IgG
- Antibody clone number
- F38-2E2
- Vial size
- 25 Tests
- Concentration
- 5 μL/Test
- Storage
- 4°C, store in dark, DO NOT FREEZE!
Submitted references High-dimensional immune cell profiling of cerebrospinal fluid from patients with metastatic breast cancer and leptomeningeal disease.
TIM-3 Expression Level on AML Blasts Correlates With Presence of Core Binding Factor Translocations Rather Than Clinical Outcomes.
Is IFN expression by NK cells a hallmark of severe COVID-19?
TIGIT(+) TIM-3(+) NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virus‑related hepatocellular carcinoma.
SLC1A1 mediated glutamine addiction and contributed to natural killer T-cell lymphoma progression with immunotherapeutic potential.
Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy.
Combined p14ARF and Interferon-β Gene Transfer to the Human Melanoma Cell Line SK-MEL-147 Promotes Oncolysis and Immune Activation.
YY1 Upregulates Checkpoint Receptors and Downregulates Type I Cytokines in Exhausted, Chronically Stimulated Human T Cells.
Low-dose interleukin-2 promotes STAT-5 phosphorylation, T(reg) survival and CTLA-4-dependent function in autoimmune liver diseases.
Alkylator-Induced and Patient-Derived Xenograft Mouse Models of Therapy-Related Myeloid Neoplasms Model Clinical Disease and Suggest the Presence of Multiple Cell Subpopulations with Leukemia Stem Cell Activity.
NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer.
Mice lacking Axl and Mer tyrosine kinase receptors are susceptible to experimental autoimmune orchitis induction.
Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy.
Anti-melanoma vaccines engineered to simultaneously modulate cytokine priming and silence PD-L1 characterized using ex vivo myeloid-derived suppressor cells as a readout of therapeutic efficacy.
TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines.
Im KW, Huppert LA, Malevanchik L, Rugo HS, Combes AJ, Campbell MJ, Krummel MF, Melisko ME
NPJ breast cancer 2023 Apr 7;9(1):22
NPJ breast cancer 2023 Apr 7;9(1):22
TIM-3 Expression Level on AML Blasts Correlates With Presence of Core Binding Factor Translocations Rather Than Clinical Outcomes.
Hong J, Xia L, Huang Z, Yuan X, Liang X, Dai J, Wu Z, Liang L, Ruan M, Long Z, Cheng X, Chen X, Ni J, Ge J, Li Q, Zeng Q, Xia R, Wang Y, Yang M
Frontiers in oncology 2022;12:879471
Frontiers in oncology 2022;12:879471
Is IFN expression by NK cells a hallmark of severe COVID-19?
Csordas BG, de Sousa Palmeira PH, Peixoto RF, Comberlang FCQDDS, de Medeiros IA, Azevedo FLAA, Veras RC, Janebro DI, Amaral IPG, Barbosa-Filho JM, Keesen TSL
Cytokine 2022 Sep;157:155971
Cytokine 2022 Sep;157:155971
TIGIT(+) TIM-3(+) NK cells are correlated with NK cell exhaustion and disease progression in patients with hepatitis B virus‑related hepatocellular carcinoma.
Yu L, Liu X, Wang X, Yan F, Wang P, Jiang Y, Du J, Yang Z
Oncoimmunology 2021;10(1):1942673
Oncoimmunology 2021;10(1):1942673
SLC1A1 mediated glutamine addiction and contributed to natural killer T-cell lymphoma progression with immunotherapeutic potential.
Xiong J, Wang N, Zhong HJ, Cui BW, Cheng S, Sun R, Chen JY, Xu PP, Cai G, Wang L, Sun XJ, Huang JY, Zhao WL
EBioMedicine 2021 Oct;72:103614
EBioMedicine 2021 Oct;72:103614
Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy.
Béziat V, Rapaport F, Hu J, Titeux M, Bonnet des Claustres M, Bourgey M, Griffin H, Bandet É, Ma CS, Sherkat R, Rokni-Zadeh H, Louis DM, Changi-Ashtiani M, Delmonte OM, Fukushima T, Habib T, Guennoun A, Khan T, Bender N, Rahman M, About F, Yang R, Rao G, Rouzaud C, Li J, Shearer D, Balogh K, Al Ali F, Ata M, Dabiri S, Momenilandi M, Nammour J, Alyanakian MA, Leruez-Ville M, Guenat D, Materna M, Marcot L, Vladikine N, Soret C, Vahidnezhad H, Youssefian L, Saeidian AH, Uitto J, Catherinot É, Navabi SS, Zarhrate M, Woodley DT, Jeljeli M, Abraham T, Belkaya S, Lorenzo L, Rosain J, Bayat M, Lanternier F, Lortholary O, Zakavi F, Gros P, Orth G, Abel L, Prétet JL, Fraitag S, Jouanguy E, Davis MM, Tangye SG, Notarangelo LD, Marr N, Waterboer T, Langlais D, Doorbar J, Hovnanian A, Christensen N, Bossuyt X, Shahrooei M, Casanova JL
Cell 2021 Jul 8;184(14):3812-3828.e30
Cell 2021 Jul 8;184(14):3812-3828.e30
Combined p14ARF and Interferon-β Gene Transfer to the Human Melanoma Cell Line SK-MEL-147 Promotes Oncolysis and Immune Activation.
Cerqueira OLD, Clavijo-Salomon MA, Cardoso EC, Citrangulo Tortelli Junior T, Mendonça SA, Barbuto JAM, Strauss BE
Frontiers in immunology 2020;11:576658
Frontiers in immunology 2020;11:576658
YY1 Upregulates Checkpoint Receptors and Downregulates Type I Cytokines in Exhausted, Chronically Stimulated Human T Cells.
Balkhi MY, Wittmann G, Xiong F, Junghans RP
iScience 2018 Apr 27;2:105-122
iScience 2018 Apr 27;2:105-122
Low-dose interleukin-2 promotes STAT-5 phosphorylation, T(reg) survival and CTLA-4-dependent function in autoimmune liver diseases.
Jeffery HC, Jeffery LE, Lutz P, Corrigan M, Webb GJ, Hirschfield GM, Adams DH, Oo YH
Clinical and experimental immunology 2017 Jun;188(3):394-411
Clinical and experimental immunology 2017 Jun;188(3):394-411
Alkylator-Induced and Patient-Derived Xenograft Mouse Models of Therapy-Related Myeloid Neoplasms Model Clinical Disease and Suggest the Presence of Multiple Cell Subpopulations with Leukemia Stem Cell Activity.
Jonas BA, Johnson C, Gratzinger D, Majeti R
PloS one 2016;11(7):e0159189
PloS one 2016;11(7):e0159189
NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer.
Karki R, Man SM, Malireddi RKS, Kesavardhana S, Zhu Q, Burton AR, Sharma BR, Qi X, Pelletier S, Vogel P, Rosenstiel P, Kanneganti TD
Nature 2016 Dec 22;540(7634):583-587
Nature 2016 Dec 22;540(7634):583-587
Mice lacking Axl and Mer tyrosine kinase receptors are susceptible to experimental autoimmune orchitis induction.
Li N, Liu Z, Zhang Y, Chen Q, Liu P, Cheng CY, Lee WM, Chen Y, Han D
Immunology and cell biology 2015 Mar;93(3):311-20
Immunology and cell biology 2015 Mar;93(3):311-20
Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy.
Qu C, Li B, Lai Y, Li H, Windust A, Hofseth LJ, Nagarkatti M, Nagarkatti P, Wang XL, Tang D, Janicki JS, Tian X, Cui T
Journal of ethnopharmacology 2015 Jun 20;168:326-36
Journal of ethnopharmacology 2015 Jun 20;168:326-36
Anti-melanoma vaccines engineered to simultaneously modulate cytokine priming and silence PD-L1 characterized using ex vivo myeloid-derived suppressor cells as a readout of therapeutic efficacy.
Liechtenstein T, Perez-Janices N, Blanco-Luquin I, Goyvaerts C, Schwarze J, Dufait I, Lanna A, Ridder M, Guerrero-Setas D, Breckpot K, Escors D
Oncoimmunology 2014;3(7):e945378
Oncoimmunology 2014;3(7):e945378
TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines.
Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK
European journal of immunology 2009 Sep;39(9):2492-501
European journal of immunology 2009 Sep;39(9):2492-501
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
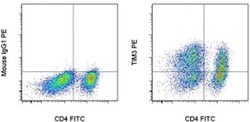
- Experimental details
- Staining of 3-day Anti-Human CD3 and CD28 Functional Grade Purifieds (Product # 16-0039-81 and Product # 16-0289-81)-stimulated normal human peripheral blood cells with Anti-Human CD4 FITC (Product # 11-0049-42) and Mouse IgG1 K Isotype Control PE (Product # 12-4714-81) (left) or Anti-Human CD366 (TIM3) PE (right). Total viable cells, as determined by Fixable Viability Dye eFluor® 660 (Product # 65-0864-14), were used for analysis.
- Conjugate
- Yellow dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
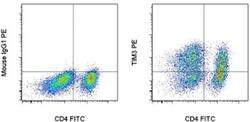
- Experimental details
- Staining of 3-day Anti-Human CD3 and CD28 Functional Grade Purifieds (Product # 16-0039-81 and Product # 16-0289-81)-stimulated normal human peripheral blood cells with Anti-Human CD4 FITC (Product # 11-0049-42) and Mouse IgG1 K Isotype Control PE (Product # 12-4714-81) (left) or Anti-Human CD366 (TIM3) PE (right). Total viable cells, as determined by Fixable Viability Dye eFluor® 660 (Product # 65-0864-14), were used for analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure2. The co-expression of TIGIT and TIM-3 is elevated on NK cells of progression patients with HBV-HCC. a-b Percentages of TIGIT + TIM-3 + NK cells on total NK cells and NK cell subsets(CD56 bright NK cells,CD56 dim NK cells, and CD56 - NK cells) from HBV-HCC, HDs, CHB and HBV-LC patients by flow cytometry analysis. c Correlation analysis of TIGIT and TIM-3 on NK cells from patients with HBV-HCC. d-e Flow-cytometry analyses (d) of TIGIT and TIM-3 were performed on PBMCs collected from HBV-HCC patients. Representative plots (e) display the expression of TIGIT + NK cells, TIM-3 + NK cells and total TIGIT + TIM-3 + NK cells from patients with progression (n = 61) and no progression (n = 72). P values were calculated by using the Kruskal-Wallis nonparametric H test (a-c). P values were obtained by the unpaired t test (d-e). * P < .05, ** P < .01, *** P < .001, **** P < .0001
- Conjugate
- Yellow dye
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
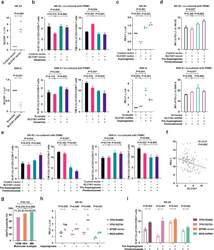
- Experimental details
- Fig. 5 Asparaginase treatment increased NKTCL cell sensitivity to anti-PD-1 antibody. (a) SLC1A1 expression on NK-92 cells transfected with SLC1A1 vector or control vector (upper panel) and SNK-6 cells transfected with SLC1A1 shRNA or scramble (lower panel). (b) Ki-67 and TIM-3 positivity of CD3+/CD8+ T cells in PBMC co-cultured with NK-92 cells (upper panel) or SNK-6 cells (lower panel) transfected with indicated vectors or shRNAs in medium with or without extra glutamine (2mM). (c) PD-L1 mRNA expression in NK-92 cells transfected with SLC1A1 vector or control vector (upper panel) and SNK-6 cells transfected with SLC1A1 shRNA or scramble (lower panel) upon asparaginase (10 IU/mL) treatment. The control vector or scramble values were normalized to 1, respectively. (d and e) Median fluorescence intensity of PD-L1 (d) on NK-92 cells (upper panel) or SNK-6 cells (lower panel), as well as Ki-67 and TIM-3 positivity of CD3+/CD8+ T cells in PBMC co-cultured with NK-92 cells (upper panel) or SNK-6 cells (lower panel) upon indicated treatment. (f) Gene expression correlation of tumor SLC1A1 with PD-L1 in NKTCL patients (n=128). (g) Tumor EAAT3 expression according to the TSIM, HEA, and MB subtypes in NKTCL patients (n=100). (h) PD-L1 mRNA expression of NK-92 cells transfected with TP53 R248Q, TP53 R273H, EP300 vector, or MGA shRNA upon indicated treatment. (i) Ki-67 positivity of CD3+/CD8+ T cells in PBMC co-cultured with NK-92 cells transfected with TP53 R248Q, TP53 R273H, EP300 vec
- Conjugate
- Yellow dye
 Explore
Explore Validate
Validate Learn
Learn Flow cytometry
Flow cytometry