Antibody data
- Antibody Data
- Antigen structure
- References [5]
- Comments [0]
- Validations
- Immunohistochemistry [2]
- Other assay [7]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-34545 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CISD2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- A suggested positive control is rat brain tissue lysate. PA5-34545 can be used with blocking peptide PEP-1588.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- Maintain refrigerated at 2-8°C for up to 3 months. For long term storage store at -20°C
Submitted references Anti-Inflammatory CDGSH Iron-Sulfur Domain 2: A Biomarker of Central Nervous System Insult in Cellular, Animal Models and Patients.
Wild Bitter Melon Exerts Anti-Inflammatory Effects by Upregulating Injury-Attenuated CISD2 Expression following Spinal Cord Injury.
CISD2 Attenuates Inflammation and Regulates Microglia Polarization in EOC Microglial Cells-As a Potential Therapeutic Target for Neurodegenerative Dementia.
Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model.
A novel CISD2 mutation associated with a classical Wolfram syndrome phenotype alters Ca2+ homeostasis and ER-mitochondria interactions.
Kung WM, Lin CC, Chen WJ, Jiang LL, Sun YY, Hsieh KH, Lin MS
Biomedicines 2022 Mar 27;10(4)
Biomedicines 2022 Mar 27;10(4)
Wild Bitter Melon Exerts Anti-Inflammatory Effects by Upregulating Injury-Attenuated CISD2 Expression following Spinal Cord Injury.
Kung WM, Lin CC, Kuo CY, Juin YC, Wu PC, Lin MS
Behavioural neurology 2020;2020:1080521
Behavioural neurology 2020;2020:1080521
CISD2 Attenuates Inflammation and Regulates Microglia Polarization in EOC Microglial Cells-As a Potential Therapeutic Target for Neurodegenerative Dementia.
Lin MS
Frontiers in aging neuroscience 2020;12:260
Frontiers in aging neuroscience 2020;12:260
Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model.
Lin CC, Chiang TH, Sun YY, Lin MS
Nutrients 2019 Mar 25;11(3)
Nutrients 2019 Mar 25;11(3)
A novel CISD2 mutation associated with a classical Wolfram syndrome phenotype alters Ca2+ homeostasis and ER-mitochondria interactions.
Rouzier C, Moore D, Delorme C, Lacas-Gervais S, Ait-El-Mkadem S, Fragaki K, Burté F, Serre V, Bannwarth S, Chaussenot A, Catala M, Yu-Wai-Man P, Paquis-Flucklinger V
Human molecular genetics 2017 May 1;26(9):1599-1611
Human molecular genetics 2017 May 1;26(9):1599-1611
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
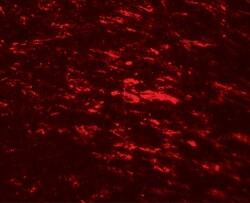
- Experimental details
- Immunofluorescence of CISD2 in rat brain tissue with CISD2 Polyclonal Antibody (Product # PA5-34545) at 20 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
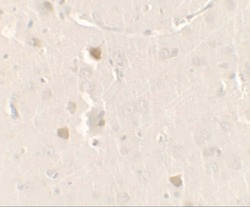
- Experimental details
- Immunohistochemistry of CISD2 in rat brain tissue with CISD2 Polyclonal Antibody (Product # PA5-34545) at 2.5 µg/mL.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
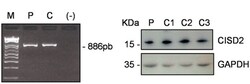
- Experimental details
- Figure 3 Absence of CISD2 RNA mis-splicing and normal CISD2 protein levels in the patient's fibroblasts. ( A ) A 886bp amplicon was obtained from cDNAs using primers in the 5'-UTR and 3'-UTR which allow the amplification of the 3 CISD2 exons. M: molecular weight marker, P: patient, C: control; (-) negative controlc. ( B ) Western blot analysis with an anti-CISD2 antibody in fibroblasts from patient (P) and 3 control individuals (C1-C3).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
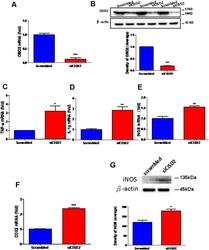
- Experimental details
- Figure 1 Knockdown of CISD2 expression augmented tendency for M1 microglial polarization and enhanced proinflammatory response in non-stressed EOC microglial cells. The conditions were set as follows: (i) scrambled RNA-transfected control cells; (ii) siCID2-transfected cells. (A) Confirmation of knockdown efficiency of CISD2 mRNA and protein (B) expression in microglia. (C) Expression of TNF-alpha mRNA, (D) IL-1beta mRNA, (E) iNOS mRNA, and (F) COX2 mRNA under all conditions. (G) iNOS protein expression in siCID2-transfected EOC microglial cells. The upper panel demonstrates the immunoblot results of iNOS at 135 kDa, and beta-actin (45 kDa) serves as an internal control; the lower panel indicates the mean (+-SEM) of iNOS/beta-actin band intensity in ratio with the control group. Vertical bars indicate the mean +- (SEM) of mRNA or protein expression ( n = 3). * p < 0.05; ** p < 0.01; *** p < 0.001 indicate statistically significant difference when compared to scrambled control cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
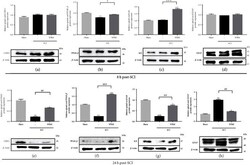
- Figure 5 WBM abolished injury-triggered GFAP and attenuated injury-downregulated CISD2, PPAR- beta , and I kappa B protein expression in mice 24 h following spinal cord hemisection in vivo . (a-d) In vivo mouse model of SCI. Results of protein expression of CISD2 (a), PPAR- beta (b), I kappa B (c), and GFAP (d) for 3 conditions 8 h after SCI. (e-h) Results of protein expression of CISD2 (e), PPAR- beta (f), I kappa B (g), and GFAP (h) for 3 conditions 24 h after SCI. The lower panel indicates representative immunoblot of the proposed protein expression, and beta -actin (42 kDa) serves as an internal control. The results shown were from one of the experiments that were repeated for at least 3 times. The upper panel indicates the mean +- SEM of the proposed protein/ beta -actin band intensity in ratio to the control group. Anti-inflammatory effects of WBM on SCI can be postulated as CISD2 preservation, and the potential upstream regulation of CISD2 on PPAR- beta , subsequent I kappa B stabilization, and therefore, NF- kappa B inhibition. Vertical bars indicate the mean +- standard error of the mean (SEM) of protein expression ( n = 3). * P < 0.05 vs. control, ** P < 0.01 vs. control, *** P < 0.001 vs. control, # P < 0.05, ## P < 0.01, and ### P < 0.001 indicate a significant difference. Pair-wise multiple comparisons between groups were performed using the Newman-Keuls method.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
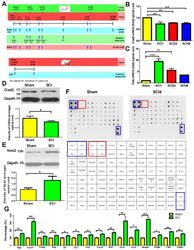
- Spinal cord injury (SCI) downregulates Cisd2 and enhances inflammatory response in the injured spinal cord of mice subjected to spinal cord hemisection. The secretion of various inflammatory mediators in the cerebrospinal fluid (CSF) of rats with SCI. ( A ) Experimental groups and the schedule of treatment with anti-CISD2 antibody in a mouse model of SCI. ( B ) The spinal cord levels of Cisd2 mRNA in the 1, 24, and 48 h post-SCI groups were significantly decreased compared with those in the sham operation group. ( C ) Under the same conditions as those in ( B ), the spinal cord levels of Tnfa mRNA were significantly increased. For B and C, vertical bars indicate mean +- standard error of mean (SEM) ( n = 6 for each group). * p < 0.05, ** p < 0.01, and *** p < 0.001 (pair-wise multiple comparisons between groups were performed using the Newman-Keuls test). The spinal cord levels of the Cisd2 protein were decreased ( D ) and those of Nos2 were increased ( E ) in the 24 h post-SCI group compared with those in the sham operation group. For D and E, the upper panel indicates representative immunoblot of the protein expression of Cisd2 (15 kDa) ( D ) and Nos2 (135 kDa); ( E ) Gapdh (35 kDa) was used as an internal control. For ( D ) and ( E ), vertical bars indicate mean +- SEM of protein levels ( n = 3 for each group). * p < 0.05, sham operation group vs. SCI groups (independent two-sample t -tests). ( F ) Detection of inflammatory mediators in the CSF using the cytokine antibody
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
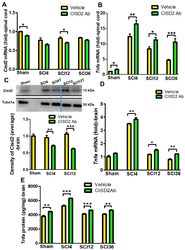
- Inhibition of Cisd2 using anti-CISD2 neutralizing antibodies exacerbated the inflammatory response in the injured spinal cord and spinal cord injury (SCI)-mediated inflamed brain of mice subjected to spinal cord hemisection. ( A ) The spinal cord levels of Cisd2 mRNA were decreased in the 4, 12, and 36 h post-SCI groups. ( B ) The differences in the spinal cord levels of Tnfa mRNA between the anti-CISD2 antibody-treated and vehicle-treated groups increased by each of these time points. ( C ) The confirmation of Cisd2 knockdown in the brain of rats subjected to spinal cord hemisection. The brain levels of Tnfa mRNA ( D ) and protein ( E ) in the anti-CISD2-treated SCI group were higher than those in the vehicle-treated SCI group at each time point. Vertical bars indicate mean +- standard error of mean of mRNA ( n = 6 for each group) and protein (for Western blot, n = 3; for enzyme-linked immunosorbent assay (ELISA), n = 6) levels. * p < 0.05, ** p < 0.01, and *** p < 0.001 (pair-wise multiple comparisons between groups were performed using the Newman-Keuls test).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
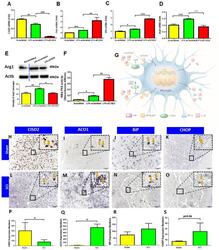
- Figure 5 siCISD2-mediated Cisd2 knockdown exacerbated the inflammatory response, promoted proinflammatory M1 phenotype, and enhanced NF-kappaB p65 nuclear translocation in EOC microglial cells. ( A ) At 8 h post-lipopolysaccharide (LPS) treatment, Cisd2 mRNA expression was decreased in the LPS-stimulated EOC microglial cells. The mRNA expression levels of Tnfa ( B ) and Il1b ( C ) were higher and the mRNA ( D ) and protein expression ( E ) levels of Arg1 (M2 microglial cell marker) were lower in the siCISD2-transfected LPS-stimulated microglial cells than in the scrambled RNA-transfected LPS-stimulated microglial cells. The upper panel in ( E ) indicates a representative immunoblot of the protein expression of Arg1 (40 kDa). Actb (45 kDa) was used as an internal control. ( F ) The results of the enzyme-linked immunosorbent assay (ELISA) revealed that LPS enhanced the DNA-binding activity of NF-kappaB p65 subunit upon Cisd2 knockdown. Vertical bars indicate mean +- standard error of mean of mRNA ( n = 3 for each group), protein ( n = 3 for each group), and ELISA ( n = 3 for each group) analysis results. * p < 0.05, ** p < 0.01, and *** p < 0.001 (pair-wise multiple comparisons between groups were performed using the Newman-Keuls test). ( G ) Schematic diagram of the mechanism underlying the regulation of the IkappaB/NF-kappaB signaling pathway by CISD2 through its anti-inflammatory effects in the microglia. The downregulation of CISD2 suppresses the inhibitory effects of CISD2
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
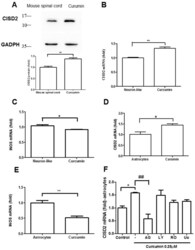
- Experimental details
- Figure 3 Curcumin upregulated CISD2 protein expression in vivo. ( A ) CISD2 protein expression in mouse spinal cords following intraperitoneal administration of curcumin at a concentration of 40 mg/kg. The upper panel presents the results of immunoblotting analysis of CISD2 (15 kDa) with GAPDH (37 kDa) serving as an internal control. The lower panel presents the mean +- (SEM) of CISD2/GAPDH band intensity as a ratio of the results from the control group (n = 4). Curcumin enhanced CISD2 mRNA expression in vitro via JAK/STAT signaling pathways. mRNA expression of CISD2 ( B ) and iNOS ( C ) in neuron-like cells (SH-SY5Y). mRNA expression levels of CISD2 ( D ) and iNOS ( E ) in astrocyte culture. ( F ) CISD2 mRNA expression following treatment with curcumin and the inhibition of JAK/STAT signaling pathways in non-stimulated astrocytes: AG490: JAK/STAT inhibitor, LY294002: PI3K inhibitor, RO318220: PKC inhibitor, U0126: MAPK inhibitor. Vertical bars indicate the mean + (SEM) of mRNA expression (n = 3). * p < 0.05, and ##,** p < 0.01 indicate differences of statistical significance.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry