Antibody data
- Antibody Data
- Antigen structure
- References [32]
- Comments [0]
- Validations
- Western blot [1]
- Immunocytochemistry [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 55054-1-AP - Provider product page

- Provider
- Proteintech Group
- Proper citation
- Proteintech Cat#55054-1-AP, RRID:AB_10792932
- Product name
- USP9X antibody
- Antibody type
- Polyclonal
- Description
- KD/KO validated USP9X antibody (Cat. #55054-1-AP) is a rabbit polyclonal antibody that shows reactivity with human, mouse, rat and has been validated for the following applications: IF, IHC, IP, WB,ELISA.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Conjugate
- Unconjugated
- Isotype
- IgG
- Vial size
- 20ul, 150ul
Submitted references Crym-positive striatal astrocytes gate perseverative behaviour.
USP46 enhances tamoxifen resistance in breast cancer cells by stabilizing PTBP1 to facilitate glycolysis.
Pharmaceutical targeting of OTUB2 sensitizes tumors to cytotoxic T cells via degradation of PD-L1.
CircMTA2 Drives Gastric Cancer Progression through Suppressing MTA2 Degradation via Interacting with UCHL3.
USP9X deubiquitinates and stabilizes CDC123 to promote breast carcinogenesis through regulating cell cycle.
BioE3 identifies specific substrates of ubiquitin E3 ligases.
Ablation of EWS-FLI1 by USP9X inhibition suppresses cancer cell growth in Ewing sarcoma.
The deubiquitinase USP9X regulates RIT1 protein abundance and oncogenic phenotypes.
Goliath induces inflammation in obese mice by linking fatty acid β-oxidation to glycolysis.
Targeting USP9X-AMPK Axis in ARID1A-Deficient Hepatocellular Carcinoma.
Induction of zinc finger protein RNF6 auto-ubiquitination for the treatment of myeloma and chronic myeloid leukemia.
USP15 regulates p66Shc stability associated with Drp1 activation in liver ischemia/reperfusion.
Deubiquitinating enzyme USP9X regulates metastasis and chemoresistance in triple-negative breast cancer by stabilizing Snail1.
TGFBR2 is a novel substrate and indirect transcription target of deubiquitylase USP9X in granulosa cells.
A Proteomic Approach for Systematic Mapping of Substrates of Human Deubiquitinating Enzymes.
Knockdown of USP9X reverses cisplatin resistance by decreasing β-catenin expression in nasopharyngeal carcinoma cells.
USP9X-mediated KDM4C deubiquitination promotes lung cancer radioresistance by epigenetically inducing TGF-β2 transcription.
Integrative oncogene-dependency mapping identifies RIT1 vulnerabilities and synergies in lung cancer.
Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival.
Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer.
Inhibition of USP9X Downregulates JAK2-V617F and Induces Apoptosis Synergistically with BH3 Mimetics Preferentially in Ruxolitinib-Persistent JAK2-V617F-Positive Leukemic Cells.
Detailed Dissection of UBE3A-Mediated DDI1 Ubiquitination.
Inhibition of the deubiquitinase USP9x induces pre-B cell homeobox 1 (PBX1) degradation and thereby stimulates prostate cancer cell apoptosis.
Deubiquitinase USP9X regulates the invasion of prostate cancer cells by regulating the ERK pathway and mitochondrial dynamics.
USP9X-mediated deubiquitination of B-cell CLL/lymphoma 9 potentiates Wnt signaling and promotes breast carcinogenesis.
The deubiquitinase USP9X promotes tumor cell survival and confers chemoresistance through YAP1 stabilization.
Deubiquitinating enzyme USP9X regulates cellular clock function by modulating the ubiquitination and degradation of a core circadian protein BMAL1.
USP9X Limits Mitotic Checkpoint Complex Turnover to Strengthen the Spindle Assembly Checkpoint and Guard against Chromosomal Instability.
Deubiquitinating Enzyme USP9X Suppresses Tumor Growth via LATS Kinase and Core Components of the Hippo Pathway.
USP9X regulates centrosome duplication and promotes breast carcinogenesis.
The X-linked deubiquitinase USP9X is an integral component of centrosome.
AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-β1 expression in male rat glomerular mesangial cells.
Ollivier M, Soto JS, Linker KE, Moye SL, Jami-Alahmadi Y, Jones AE, Divakaruni AS, Kawaguchi R, Wohlschlegel JA, Khakh BS
Nature 2024 Mar;627(8003):358-366
Nature 2024 Mar;627(8003):358-366
USP46 enhances tamoxifen resistance in breast cancer cells by stabilizing PTBP1 to facilitate glycolysis.
Gao S, Wang Y, Xu Y, Liu L, Liu S
Biochimica et biophysica acta. Molecular basis of disease 2024 Mar;1870(3):167011
Biochimica et biophysica acta. Molecular basis of disease 2024 Mar;1870(3):167011
Pharmaceutical targeting of OTUB2 sensitizes tumors to cytotoxic T cells via degradation of PD-L1.
Ren W, Xu Z, Chang Y, Ju F, Wu H, Liang Z, Zhao M, Wang N, Lin Y, Xu C, Chen S, Rao Y, Lin C, Yang J, Liu P, Zhang J, Huang C, Xia N
Nature communications 2024 Jan 2;15(1):9
Nature communications 2024 Jan 2;15(1):9
CircMTA2 Drives Gastric Cancer Progression through Suppressing MTA2 Degradation via Interacting with UCHL3.
Xie G, Lei B, Yin Z, Xu F, Liu X
International journal of molecular sciences 2024 Feb 29;25(5)
International journal of molecular sciences 2024 Feb 29;25(5)
USP9X deubiquitinates and stabilizes CDC123 to promote breast carcinogenesis through regulating cell cycle.
Song N, Deng L, Zeng L, He L, Liu C, Liu L, Fu R
Molecular carcinogenesis 2023 Oct;62(10):1487-1503
Molecular carcinogenesis 2023 Oct;62(10):1487-1503
BioE3 identifies specific substrates of ubiquitin E3 ligases.
Barroso-Gomila O, Merino-Cacho L, Muratore V, Perez C, Taibi V, Maspero E, Azkargorta M, Iloro I, Trulsson F, Vertegaal ACO, Mayor U, Elortza F, Polo S, Barrio R, Sutherland JD
Nature communications 2023 Nov 23;14(1):7656
Nature communications 2023 Nov 23;14(1):7656
Ablation of EWS-FLI1 by USP9X inhibition suppresses cancer cell growth in Ewing sarcoma.
Wang S, Huo X, Yang Y, Mo Y, Kollipara RK, Kittler R
Cancer letters 2023 Jan 1;552:215984
Cancer letters 2023 Jan 1;552:215984
The deubiquitinase USP9X regulates RIT1 protein abundance and oncogenic phenotypes.
Riley AK, Grant M, Snell A, Vichas A, Moorthi S, Urisman A, Castel P, Wan L, Berger AH
bioRxiv : the preprint server for biology 2023 Dec 1;
bioRxiv : the preprint server for biology 2023 Dec 1;
Goliath induces inflammation in obese mice by linking fatty acid β-oxidation to glycolysis.
Hao S, Zhang S, Ye J, Chen L, Wang Y, Pei S, Zhu Q, Xu J, Tao Y, Zhou N, Yin H, Duan CW, Mao C, Zheng M, Xiao Y
EMBO reports 2023 Apr 5;24(4):e56932
EMBO reports 2023 Apr 5;24(4):e56932
Targeting USP9X-AMPK Axis in ARID1A-Deficient Hepatocellular Carcinoma.
Zhang FK, Ni QZ, Wang K, Cao HJ, Guan DX, Zhang EB, Ma N, Wang YK, Zheng QW, Xu S, Zhu B, Chen TW, Xia J, Qiu XS, Ding XF, Jiang H, Qiu L, Wang X, Chen W, Cheng SQ, Xie D, Li JJ
Cellular and molecular gastroenterology and hepatology 2022;14(1):101-127
Cellular and molecular gastroenterology and hepatology 2022;14(1):101-127
Induction of zinc finger protein RNF6 auto-ubiquitination for the treatment of myeloma and chronic myeloid leukemia.
Zhuang H, Ren Y, Mao C, Zhong Y, Zhang Z, Cao B, Zhang Y, Huang J, Xu G, Huang Z, Xu Y, Mao X
The Journal of biological chemistry 2022 Sep;298(9):102314
The Journal of biological chemistry 2022 Sep;298(9):102314
USP15 regulates p66Shc stability associated with Drp1 activation in liver ischemia/reperfusion.
Tian X, Zhao Y, Yang Z, Lu Q, Zhou L, Zheng S
Cell death & disease 2022 Sep 26;13(9):823
Cell death & disease 2022 Sep 26;13(9):823
Deubiquitinating enzyme USP9X regulates metastasis and chemoresistance in triple-negative breast cancer by stabilizing Snail1.
Guan T, Yang X, Liang H, Chen J, Chen Y, Zhu Y, Liu T
Journal of cellular physiology 2022 Jul;237(7):2992-3000
Journal of cellular physiology 2022 Jul;237(7):2992-3000
TGFBR2 is a novel substrate and indirect transcription target of deubiquitylase USP9X in granulosa cells.
Yang L, Wang S, Pan Z, Du X, Li Q
Journal of cellular physiology 2022 Jul;237(7):2969-2979
Journal of cellular physiology 2022 Jul;237(7):2969-2979
A Proteomic Approach for Systematic Mapping of Substrates of Human Deubiquitinating Enzymes.
Ramirez J, Prieto G, Olazabal-Herrero A, Borràs E, Fernandez-Vigo E, Alduntzin U, Osinalde N, Beaskoetxea J, Lectez B, Aloria K, Rodriguez JA, Paradela A, Sabidó E, Muñoz J, Corrales F, Arizmendi JM, Mayor U
International journal of molecular sciences 2021 May 3;22(9)
International journal of molecular sciences 2021 May 3;22(9)
Knockdown of USP9X reverses cisplatin resistance by decreasing β-catenin expression in nasopharyngeal carcinoma cells.
Lu WX, Gan GS, Yang B
Neoplasma 2021 Jul;68(4):810-822
Neoplasma 2021 Jul;68(4):810-822
USP9X-mediated KDM4C deubiquitination promotes lung cancer radioresistance by epigenetically inducing TGF-β2 transcription.
Jie X, Fong WP, Zhou R, Zhao Y, Zhao Y, Meng R, Zhang S, Dong X, Zhang T, Yang K, Wu G, Xu S
Cell death and differentiation 2021 Jul;28(7):2095-2111
Cell death and differentiation 2021 Jul;28(7):2095-2111
Integrative oncogene-dependency mapping identifies RIT1 vulnerabilities and synergies in lung cancer.
Vichas A, Riley AK, Nkinsi NT, Kamlapurkar S, Parrish PCR, Lo A, Duke F, Chen J, Fung I, Watson J, Rees M, Gabel AM, Thomas JD, Bradley RK, Lee JK, Hatch EM, Baine MK, Rekhtman N, Ladanyi M, Piccioni F, Berger AH
Nature communications 2021 Aug 9;12(1):4789
Nature communications 2021 Aug 9;12(1):4789
Antagonistic activities of CDC14B and CDK1 on USP9X regulate WT1-dependent mitotic transcription and survival.
Dietachmayr M, Rathakrishnan A, Karpiuk O, von Zweydorf F, Engleitner T, Fernández-Sáiz V, Schenk P, Ueffing M, Rad R, Eilers M, Gloeckner CJ, Clemm von Hohenberg K, Bassermann F
Nature communications 2020 Mar 9;11(1):1268
Nature communications 2020 Mar 9;11(1):1268
Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer.
Liu J, Feng J, Li L, Lin L, Ji J, Lin C, Liu L, Zhang N, Duan D, Li Z, Huang B, Zhang Y, Lu J
EMBO reports 2020 Feb 5;21(2):e48597
EMBO reports 2020 Feb 5;21(2):e48597
Inhibition of USP9X Downregulates JAK2-V617F and Induces Apoptosis Synergistically with BH3 Mimetics Preferentially in Ruxolitinib-Persistent JAK2-V617F-Positive Leukemic Cells.
Akiyama H, Umezawa Y, Watanabe D, Okada K, Ishida S, Nogami A, Miura O
Cancers 2020 Feb 10;12(2)
Cancers 2020 Feb 10;12(2)
Detailed Dissection of UBE3A-Mediated DDI1 Ubiquitination.
Elu N, Osinalde N, Beaskoetxea J, Ramirez J, Lectez B, Aloria K, Rodriguez JA, Arizmendi JM, Mayor U
Frontiers in physiology 2019;10:534
Frontiers in physiology 2019;10:534
Inhibition of the deubiquitinase USP9x induces pre-B cell homeobox 1 (PBX1) degradation and thereby stimulates prostate cancer cell apoptosis.
Liu Y, Xu X, Lin P, He Y, Zhang Y, Cao B, Zhang Z, Sethi G, Liu J, Zhou X, Mao X
The Journal of biological chemistry 2019 Mar 22;294(12):4572-4582
The Journal of biological chemistry 2019 Mar 22;294(12):4572-4582
Deubiquitinase USP9X regulates the invasion of prostate cancer cells by regulating the ERK pathway and mitochondrial dynamics.
Zhang J, Wang J, Luan T, Zuo Y, Chen J, Zhang H, Ye Z, Wang H, Hai B
Oncology reports 2019 Jun;41(6):3292-3304
Oncology reports 2019 Jun;41(6):3292-3304
USP9X-mediated deubiquitination of B-cell CLL/lymphoma 9 potentiates Wnt signaling and promotes breast carcinogenesis.
Shang Z, Zhao J, Zhang Q, Cao C, Tian S, Zhang K, Liu L, Shi L, Yu N, Yang S
The Journal of biological chemistry 2019 Jun 21;294(25):9844-9857
The Journal of biological chemistry 2019 Jun 21;294(25):9844-9857
The deubiquitinase USP9X promotes tumor cell survival and confers chemoresistance through YAP1 stabilization.
Li L, Liu T, Li Y, Wu C, Luo K, Yin Y, Chen Y, Nowsheen S, Wu J, Lou Z, Yuan J
Oncogene 2018 May;37(18):2422-2431
Oncogene 2018 May;37(18):2422-2431
Deubiquitinating enzyme USP9X regulates cellular clock function by modulating the ubiquitination and degradation of a core circadian protein BMAL1.
Zhang Y, Duan C, Yang J, Chen S, Liu Q, Zhou L, Huang Z, Xu Y, Xu G
The Biochemical journal 2018 Apr 30;475(8):1507-1522
The Biochemical journal 2018 Apr 30;475(8):1507-1522
USP9X Limits Mitotic Checkpoint Complex Turnover to Strengthen the Spindle Assembly Checkpoint and Guard against Chromosomal Instability.
Skowyra A, Allan LA, Saurin AT, Clarke PR
Cell reports 2018 Apr 17;23(3):852-865
Cell reports 2018 Apr 17;23(3):852-865
Deubiquitinating Enzyme USP9X Suppresses Tumor Growth via LATS Kinase and Core Components of the Hippo Pathway.
Toloczko A, Guo F, Yuen HF, Wen Q, Wood SA, Ong YS, Chan PY, Shaik AA, Gunaratne J, Dunne MJ, Hong W, Chan SW
Cancer research 2017 Sep 15;77(18):4921-4933
Cancer research 2017 Sep 15;77(18):4921-4933
USP9X regulates centrosome duplication and promotes breast carcinogenesis.
Li X, Song N, Liu L, Liu X, Ding X, Song X, Yang S, Shan L, Zhou X, Su D, Wang Y, Zhang Q, Cao C, Ma S, Yu N, Yang F, Wang Y, Yao Z, Shang Y, Shi L
Nature communications 2017 Mar 31;8:14866
Nature communications 2017 Mar 31;8:14866
The X-linked deubiquitinase USP9X is an integral component of centrosome.
Wang Q, Tang Y, Xu Y, Xu S, Jiang Y, Dong Q, Zhou Y, Ge W
The Journal of biological chemistry 2017 Aug 4;292(31):12874-12884
The Journal of biological chemistry 2017 Aug 4;292(31):12874-12884
AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-β1 expression in male rat glomerular mesangial cells.
Huang KP, Chen C, Hao J, Huang JY, Liu PQ, Huang HQ
Endocrinology 2015 Jan;156(1):268-79
Endocrinology 2015 Jan;156(1):268-79
No comments: Submit comment
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image

- Experimental details
- HEK-293 cells were subjected to SDS PAGE followed by western blot with 55054-1-AP(USP9X antibody) at dilution of 1:500
- Sample type
- cell line
Supportive validation
- Submitted by
- Proteintech Group (provider)
- Main image
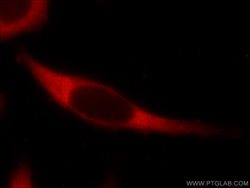
- Experimental details
- The USP9X antibody from Proteintech is a rabbit polyclonal antibody to a peptide of human USP9X. This antibody recognizes human,mouse,rat antigen. The USP9X antibody has been validated for the following applications: ELISA, WB, IF, IHC analysis.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot ELISA
ELISA