Antibody data
- Antibody Data
- Antigen structure
- References [32]
- Comments [0]
- Validations
- Immunohistochemistry [2]
- Other assay [31]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-915 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PMCA2 ATPase Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-915 detects calcium pump of the plasma membrane 2 (PMCA2 ATPase) from human and rat and porcine tissues and cells. PA1-915 has been successfully used in Western blot procedures. By Western blot, this antibody detects an ~127 kDa protein and an ~133 kDa protein representing PMCA2a and PMCA2b ATPase, respectively, from rat brain microsomal fractions. PA1-915 immunizing peptide corresponds to amino acid residues 5-19 from human PMCA2 ATPase protein. This sequence is completely conserved between human and rat PMCA2 ATPase.
- Reactivity
- Human, Rat, Porcine
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references MAL2 mediates the formation of stable HER2 signaling complexes within lipid raft-rich membrane protrusions in breast cancer cells.
Unbiased yeast screens identify cellular pathways affected in Niemann-Pick disease type C.
Plasma Membrane Ca(2+) ATPase Isoform 4 (PMCA4) Has an Important Role in Numerous Hallmarks of Pancreatic Cancer.
Dynamic profile of EVs in porcine oviductal fluid during the periovulatory period.
Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents.
HER2 signaling regulates HER2 localization and membrane retention.
Glutamate Deregulation in Ketamine-Induced Psychosis-A Potential Role of PSD95, NMDA Receptor and PMCA Interaction.
Sphingomyelin-induced inhibition of the plasma membrane calcium ATPase causes neurodegeneration in type A Niemann-Pick disease.
Early growth response protein 1 regulates promoter activity of α-plasma membrane calcium ATPase 2, a major calcium pump in the brain and auditory system.
Maintenance of neuronal size gradient in MNTB requires sound-evoked activity.
The scaffolding protein NHERF1 regulates the stability and activity of the tyrosine kinase HER2.
The calcium pump plasma membrane Ca(2+)-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin.
PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer.
Changes in cochlear PMCA2 expression correlate with the maturation of auditory sensitivity.
A new Atp2b2 deafwaddler allele, dfw(i5), interacts strongly with Cdh23 and other auditory modifiers.
PMCA2 via PSD-95 controls calcium signaling by α7-containing nicotinic acetylcholine receptors on aspiny interneurons.
Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism.
The development, distribution and density of the plasma membrane calcium ATPase 2 calcium pump in rat cochlear hair cells.
Interaction of plasma membrane Ca(2+)-ATPase isoform 4 with calcineurin A: implications for catecholamine secretion by PC12 cells.
The novel PMCA2 pump mutation Tommy impairs cytosolic calcium clearance in hair cells and links to deafness in mice.
Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca(2+)-ATPase.
Compartment-specific regulation of plasma membrane calcium ATPase type 2 in the chick auditory brainstem.
Localization of plasma membrane and secretory calcium pumps in the mammary gland.
The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk.
Differential regulation of the apical plasma membrane Ca(2+) -ATPase by protein kinase A in parotid acinar cells.
Plasma membrane calcium pumps in mouse olfactory sensory neurons.
The plasma membrane Ca2+-ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes.
Expression and immunolocalization of plasma membrane calcium ATPase isoforms in human corneal epithelium.
Regional distribution of Na,K-ATPase activity in porcine lens epithelium.
Regional distribution of Na,K-ATPase activity in porcine lens epithelium.
Developmental assembly of transduction apparatus in chick basilar papilla.
Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies.
Jeong J, Shin JH, Li W, Hong JY, Lim J, Hwang JY, Chung JJ, Yan Q, Liu Y, Choi J, Wysolmerski J
Cell reports 2021 Dec 28;37(13):110160
Cell reports 2021 Dec 28;37(13):110160
Unbiased yeast screens identify cellular pathways affected in Niemann-Pick disease type C.
Colaco A, Fernández-Suárez ME, Shepherd D, Gal L, Bibi C, Chuartzman S, Diot A, Morten K, Eden E, Porter FD, Poulton J, Platt N, Schuldiner M, Platt FM
Life science alliance 2020 Jul;3(7)
Life science alliance 2020 Jul;3(7)
Plasma Membrane Ca(2+) ATPase Isoform 4 (PMCA4) Has an Important Role in Numerous Hallmarks of Pancreatic Cancer.
Sritangos P, Pena Alarcon E, James AD, Sultan A, Richardson DA, Bruce JIE
Cancers 2020 Jan 16;12(1)
Cancers 2020 Jan 16;12(1)
Dynamic profile of EVs in porcine oviductal fluid during the periovulatory period.
Laezer I, Palma-Vera SE, Liu F, Frank M, Trakooljul N, Vernunft A, Schoen J, Chen S
Reproduction (Cambridge, England) 2020 Apr;159(4):371-382
Reproduction (Cambridge, England) 2020 Apr;159(4):371-382
Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents.
Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B, Hu YJ, Hu JH, Thompson DB, Shu Y, Li Y, Wang H, Yang S, Xu Q, Polley DB, Liberman MC, Kong WJ, Holt JR, Chen ZY, Liu DR
Nature 2018 Jan 11;553(7687):217-221
Nature 2018 Jan 11;553(7687):217-221
HER2 signaling regulates HER2 localization and membrane retention.
Jeong J, Kim W, Kim LK, VanHouten J, Wysolmerski JJ
PloS one 2017;12(4):e0174849
PloS one 2017;12(4):e0174849
Glutamate Deregulation in Ketamine-Induced Psychosis-A Potential Role of PSD95, NMDA Receptor and PMCA Interaction.
Lisek M, Ferenc B, Studzian M, Pulaski L, Guo F, Zylinska L, Boczek T
Frontiers in cellular neuroscience 2017;11:181
Frontiers in cellular neuroscience 2017;11:181
Sphingomyelin-induced inhibition of the plasma membrane calcium ATPase causes neurodegeneration in type A Niemann-Pick disease.
Pérez-Cañamás A, Benvegnù S, Rueda CB, Rábano A, Satrústegui J, Ledesma MD
Molecular psychiatry 2017 May;22(5):711-723
Molecular psychiatry 2017 May;22(5):711-723
Early growth response protein 1 regulates promoter activity of α-plasma membrane calcium ATPase 2, a major calcium pump in the brain and auditory system.
Minich RR, Li J, Tempel BL
BMC molecular biology 2017 May 22;18(1):14
BMC molecular biology 2017 May 22;18(1):14
Maintenance of neuronal size gradient in MNTB requires sound-evoked activity.
Weatherstone JH, Kopp-Scheinpflug C, Pilati N, Wang Y, Forsythe ID, Rubel EW, Tempel BL
Journal of neurophysiology 2017 Feb 1;117(2):756-766
Journal of neurophysiology 2017 Feb 1;117(2):756-766
The scaffolding protein NHERF1 regulates the stability and activity of the tyrosine kinase HER2.
Jeong J, VanHouten JN, Kim W, Dann P, Sullivan C, Choi J, Sneddon WB, Friedman PA, Wysolmerski JJ
The Journal of biological chemistry 2017 Apr 21;292(16):6555-6568
The Journal of biological chemistry 2017 Apr 21;292(16):6555-6568
The calcium pump plasma membrane Ca(2+)-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin.
Peters AA, Milevskiy MJ, Lee WC, Curry MC, Smart CE, Saunus JM, Reid L, da Silva L, Marcial DL, Dray E, Brown MA, Lakhani SR, Roberts-Thomson SJ, Monteith GR
Scientific reports 2016 May 5;6:25505
Scientific reports 2016 May 5;6:25505
PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer.
Jeong J, VanHouten JN, Dann P, Kim W, Sullivan C, Yu H, Liotta L, Espina V, Stern DF, Friedman PA, Wysolmerski JJ
Proceedings of the National Academy of Sciences of the United States of America 2016 Jan 19;113(3):E282-90
Proceedings of the National Academy of Sciences of the United States of America 2016 Jan 19;113(3):E282-90
Changes in cochlear PMCA2 expression correlate with the maturation of auditory sensitivity.
Watson CJ, Lies SM, Minich RR, Tempel BL
Journal of the Association for Research in Otolaryngology : JARO 2014 Aug;15(4):543-54
Journal of the Association for Research in Otolaryngology : JARO 2014 Aug;15(4):543-54
A new Atp2b2 deafwaddler allele, dfw(i5), interacts strongly with Cdh23 and other auditory modifiers.
Watson CJ, Tempel BL
Hearing research 2013 Oct;304:41-8
Hearing research 2013 Oct;304:41-8
PMCA2 via PSD-95 controls calcium signaling by α7-containing nicotinic acetylcholine receptors on aspiny interneurons.
Gómez-Varela D, Schmidt M, Schoellerman J, Peters EC, Berg DK
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 May 16;32(20):6894-905
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 May 16;32(20):6894-905
Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism.
Yang J, Bouvron S, Lv P, Chi F, Yamoah EN
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Mar 14;32(11):3712-25
The Journal of neuroscience : the official journal of the Society for Neuroscience 2012 Mar 14;32(11):3712-25
The development, distribution and density of the plasma membrane calcium ATPase 2 calcium pump in rat cochlear hair cells.
Chen Q, Mahendrasingam S, Tickle JA, Hackney CM, Furness DN, Fettiplace R
The European journal of neuroscience 2012 Aug;36(3):2302-10
The European journal of neuroscience 2012 Aug;36(3):2302-10
Interaction of plasma membrane Ca(2+)-ATPase isoform 4 with calcineurin A: implications for catecholamine secretion by PC12 cells.
Kosiorek M, Podszywalow-Bartnicka P, Zylinska L, Zablocki K, Pikula S
Biochemical and biophysical research communications 2011 Jul 29;411(2):235-40
Biochemical and biophysical research communications 2011 Jul 29;411(2):235-40
The novel PMCA2 pump mutation Tommy impairs cytosolic calcium clearance in hair cells and links to deafness in mice.
Bortolozzi M, Brini M, Parkinson N, Crispino G, Scimemi P, De Siati RD, Di Leva F, Parker A, Ortolano S, Arslan E, Brown SD, Carafoli E, Mammano F
The Journal of biological chemistry 2010 Nov 26;285(48):37693-703
The Journal of biological chemistry 2010 Nov 26;285(48):37693-703
Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca(2+)-ATPase.
Zaidi A, Fernandes D, Bean JL, Michaelis ML
Free radical biology & medicine 2009 Nov 15;47(10):1507-14
Free radical biology & medicine 2009 Nov 15;47(10):1507-14
Compartment-specific regulation of plasma membrane calcium ATPase type 2 in the chick auditory brainstem.
Wang Y, Cunningham DE, Tempel BL, Rubel EW
The Journal of comparative neurology 2009 Jun 20;514(6):624-40
The Journal of comparative neurology 2009 Jun 20;514(6):624-40
Localization of plasma membrane and secretory calcium pumps in the mammary gland.
Faddy HM, Smart CE, Xu R, Lee GY, Kenny PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ, Monteith GR
Biochemical and biophysical research communications 2008 May 9;369(3):977-81
Biochemical and biophysical research communications 2008 May 9;369(3):977-81
The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk.
VanHouten JN, Neville MC, Wysolmerski JJ
Endocrinology 2007 Dec;148(12):5943-54
Endocrinology 2007 Dec;148(12):5943-54
Differential regulation of the apical plasma membrane Ca(2+) -ATPase by protein kinase A in parotid acinar cells.
Baggaley E, McLarnon S, Demeter I, Varga G, Bruce JI
The Journal of biological chemistry 2007 Dec 28;282(52):37678-93
The Journal of biological chemistry 2007 Dec 28;282(52):37678-93
Plasma membrane calcium pumps in mouse olfactory sensory neurons.
Weeraratne SD, Valentine M, Cusick M, Delay R, Van Houten JL
Chemical senses 2006 Oct;31(8):725-30
Chemical senses 2006 Oct;31(8):725-30
The plasma membrane Ca2+-ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes.
Sepúlveda MR, Berrocal-Carrillo M, Gasset M, Mata AM
The Journal of biological chemistry 2006 Jan 6;281(1):447-53
The Journal of biological chemistry 2006 Jan 6;281(1):447-53
Expression and immunolocalization of plasma membrane calcium ATPase isoforms in human corneal epithelium.
Talarico EF Jr, Kennedy BG, Marfurt CF, Loeffler KU, Mangini NJ
Molecular vision 2005 Mar 2;11:169-78
Molecular vision 2005 Mar 2;11:169-78
Regional distribution of Na,K-ATPase activity in porcine lens epithelium.
Tamiya S, Dean WL, Paterson CA, Delamere NA
Investigative ophthalmology & visual science 2003 Oct;44(10):4395-9
Investigative ophthalmology & visual science 2003 Oct;44(10):4395-9
Regional distribution of Na,K-ATPase activity in porcine lens epithelium.
Tamiya S, Dean WL, Paterson CA, Delamere NA
Investigative ophthalmology & visual science 2003 Oct;44(10):4395-9
Investigative ophthalmology & visual science 2003 Oct;44(10):4395-9
Developmental assembly of transduction apparatus in chick basilar papilla.
Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN
The Journal of neuroscience : the official journal of the Society for Neuroscience 2003 Nov 26;23(34):10815-26
The Journal of neuroscience : the official journal of the Society for Neuroscience 2003 Nov 26;23(34):10815-26
Detection of isoform 4 of the plasma membrane calcium pump in human tissues by using isoform-specific monoclonal antibodies.
Caride AJ, Filoteo AG, Enyedi A, Verma AK, Penniston JT
The Biochemical journal 1996 May 15;316 ( Pt 1):353-9
The Biochemical journal 1996 May 15;316 ( Pt 1):353-9
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
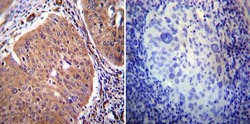
- Experimental details
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human cervical carcinoma tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a rabbit polyclonal antibody recognizing PMCA2 ATPase (Product # PA1-915) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
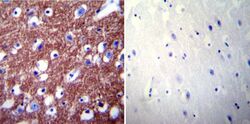
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Human brain tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a rabbit polyclonal antibody recognizing PMCA2 ATPase (Product # PA1-915) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
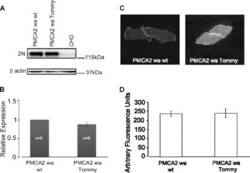
- FIGURE 4. Expression and immunolocalization of recombinant PMCA2 w/a pumps in CHO cells. A , Western blotting analysis is shown. The band of ~130 kDa corresponds to PMCA2, and the band of ~42 kDa corresponds to beta-actin. B , densitometric analysis of relative expression of wild type PMCA2 w/a and PMCA2 w/a harboring the Tommy mutation is shown. The amounts of pump protein were as normalized for beta-actin. C , immunolocalization of wild type ( left ) and mutant (right ) PMCA2 w/a in transiently transfected CHO cells is shown. The interaction with the 2N antibody was probed by the AlexaFluor488-conjugated secondary antibody. D , fluorescence levels in the plasma membrane is quantified as described under ""Experimental Procedures."" Bars represent S.D.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
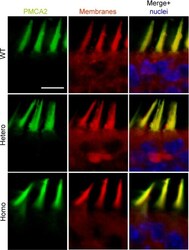
- FIGURE 7. PMCA2 immunolabeling in neonatal utricle. Confocal imaging immunoassays of PMCA2 (stained by Alexa 488 conjugated, green ) in P7 utricle cultures from wild type, heterozygous, and homozygous Tommy mice (for details see ""Experimental Procedures""). Membranes ( red ) are stained by FM4-64FX, whereas nuclei ( blue ) are stained by DAPI. Scale bar , 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
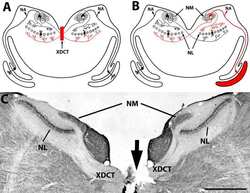
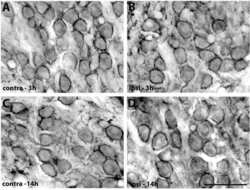
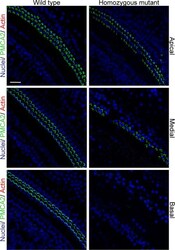
- FIGURE 8. PMCA2 immunolabeling in P60 cochlea whole mounts. Horizontal sections (orthogonal to the modiolus) of cochlea whole mounts from P60 wild type ( left column ) and homozygous ( right column ) Tommy mice are shown. Images from apical, medial, and basal regions are obtained by maximal back-projection of 20 confocal optical sections from a 2-mum step z-stack of wild type and homozygous Tommy mice. PMCA2 expression was probed by a PMCA2 selective antibody (Alexa 488 conjugated, green ), and nuclei were stained with DAPI ( blue ). Scale bar , 15 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
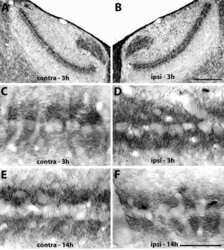
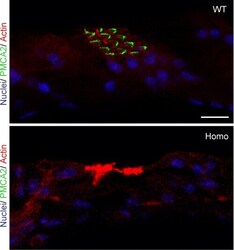
- FIGURE 9. PMCA2 immunolabeling in basal cochlear transversal sections (parallel to the modiolus) of P60 Tommy mice. Images were obtained by maximal back-projection of 2-6 confocal optical sections from a 1-mum step z-stack of wild type ( top panel ) and homozygous ( bottom panel ) Tommy mice. Actin filaments were stained with phalloidin (Texas Red-conjugated, red ) and nuclei with DAPI ( blue ). Scale bar , 15 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
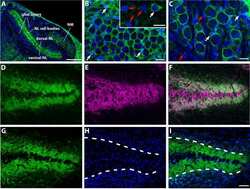
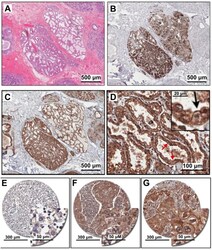
- Figure 1 PMCA2 expression in human breast tissue with lactational change and in breast cancer tissues. ( A - D ) Human breast sections with lactational change; ( A ) hematoxylin and eosin staining; ( B ) beta-casein staining showing accumulation of milk protein in the lumen; ( C , D ) PMCA2 staining showing PMCA2 accumulation on apical membranes of epithelial cells with lactational change. ( E - G ) Examples of PMCA2 staining in breast cancer tissues; ( E ) PMCA2-negative cancer, showing light diffuse brown background staining, ( F ) More intense PMCA2 staining in the cytoplasm with no plasma membrane localization, these were also defined as PMCA2-negative cancer in our analysis, ( G ) Clear PMCA2 staining on the plasma membrane, these were defined as PMCA2-positive cancers (PMCA2-PM+) given the localization of the PMCA2 plasma membrane Ca 2+ pump on the plasma membrane of these breast cancer cells. Original magnification 3x ( A-C ), 20x ( D ), 5x main images and 20x for insert ( E - G ).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
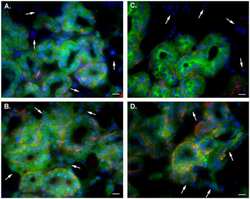
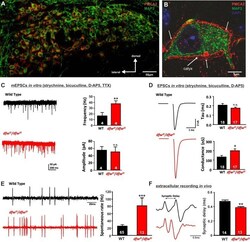
- Fig. 1. PMCA2 regulates transmitter release in the MNTB. A and B : immunohistochemical labeling for MAP2 and PMCA2 in the MNTB ( A ) and in an individual MNTB neuron ( B ). A cross section through the calyx is marked ""calyx."" Arrows show where PMCA2 appears to be localized presynaptically in the outer membrane of the calyx. C : voltage-clamp recordings from postsynaptic MNTB neurons in acute brain slices show a higher frequency of miniature excitatory postsynaptic currents (mEPSCs) in the MNTB of dfw 2J / dfw 2J mice compared with wild type (WT). D : calyceal EPSCs evoked by midline stimulation are larger in dfw 2J / dfw 2J mice compared with WT. Stimulus artifacts have been deleted for clarity. WT data include 7 medial cells, 3 lateral cells, and 8 cells with no information about location in the MNTB. dfw 2J / dfw 2J data include 7 medial cells, 6 lateral cells, and 4 cells with no information about location in the MNTB (see also Fig. 4 C ). E and F : in vivo single-unit recordings of MNTB neurons measured higher spontaneous firing rates ( E ) and shorter synaptic delays ( F ) in dfw 2J / dfw 2J mice compared with WT. *** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
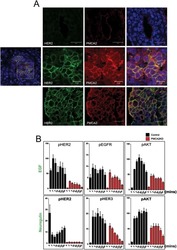
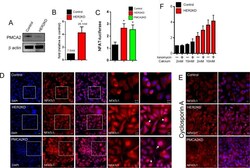
- Fig 3 HER2 regulates intracellular calcium concentration in SKBR3 cells. A) PMCA2 levels in control versus HER2KD-SKBR3 cells as assessed by immunoblot. B) Intracellular calcium measurements in HER2KD-SKBR3 cells relative to control. Numbers indicate the mean calcium concentrations estimated by FURA2 measurements. Each bar represents the mean +- SEM of 3 separate experiments. Asterisk denotes statistically significant difference. C) Expression of a NFAT-luciferase indicator construct in control SKBR3 cells, HER2KD-SKBR3 cells and PMCA2KD-SKBR3 cells. Each bar represents the mean +- SEM of 3 separate experiments. Asterisks denote statistically significant differences. D) Con-focal images of immunofluorescence for NFATc1 (red) or DAPI (blue) in control (top row), HER2KD-SKBR3 (middle row) or PMCA2KD-SKBR3 (bottom row) cells. Scale bars represent 10mum. E) Apoptosis as assessed by TUNEL assay in HER2KD-SKBR3 cells relative to controls exposed to differing concentrations of extracellular calcium +- ionomycin. Each bar represents the mean +- SEM of 3 separate experiments. F) Con-focal images of immunofluorescence for NFATc1 (red) or DAPI (blue) in control (top row), HER2KD-SKBR3 (middle row) or PMCA2KD-SKBR3 (bottom row) cells treated with cyclosporine A to block calcineurin activity. Scale bars represent 10mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
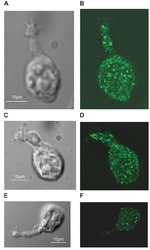
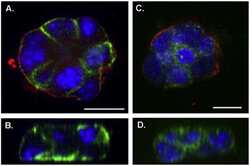
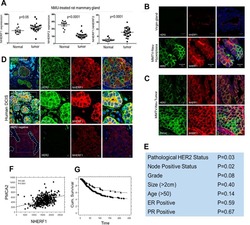
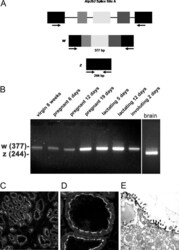
- Fig. 2. A, The genomic organization of the Atp2b2 (PMCA2) gene at splice site A is illustrated with the alternatively spliced variants PMCA2w and PMCA2z shown below. RT-PCR was performed with primers (represented by arrows ) flanking the splice site. B, RT-PCR was performed on RNA from mammary glands of mice at the developmental stages noted in the figure. Mouse brain RNA served as a control for the PMCA2z splice variant (the brain sample was run on a different gel). PCR products were of the predicted sizes for either the w splice variant (377 bp) or the PMCA2z splice variant (244 bp), but PCR products were sequenced to confirm their identity. Throughout postnatal development, the mouse mammary gland expresses only the PMCA2w variant of splice site A. C and D, Immunofluorescent staining for PMCA2 in mammary tissue from a lactating mouse (d 12) demonstrated that it was located at the apical plasma membrane. E, An electron micrograph of lactating mammary tissue stained for PMCA2 using immunoperoxidase techniques confirmed the apical membrane localization. There was no PMCA2 staining in secretory vesicles or other intracellular compartments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
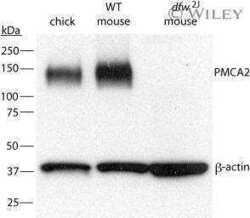
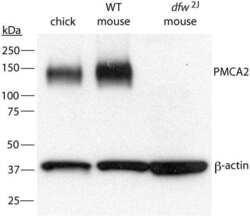
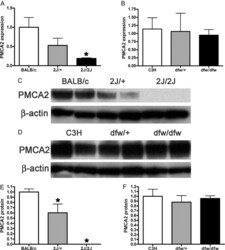
- Fig. 3. A, QRT-PCR demonstrates a progressive reduction in the expression of the PMCA2 gene ( Atp2b2 ) in the mammary glands of dfw-2J/+ mice and dfw-2J/dfw-2J mice compared with wild-type BALB/c mice. PMCA2 mRNA levels were reduced by 50% in Dfw-2J/+ glands and by 85% in dfw-2J/dfw-2J glands. B, In contrast, PMCA2 mRNA levels in mice with the dfw mutation (dfw/+ and dfw/dfw) were not different from those in control C3H mice. C, Western blotting for PMCA2 in the plasma membrane fraction of lactating mouse mammary glands revealed abundant PMCA2 in BALB/c mice (lanes 1 and 2), reduced PMCA2 in dfw-2J/+ mice (lanes 3 and 4), and no PMCA2 in dfw-2J/dfw-2J mice (lanes 5 and 6). D, PMCA2 protein levels were similar in C3H (lanes 1 and 2), dfw/+ (lanes 3 and 4), and dfw/dfw (lanes 5 and 6) mice. E and F, Quantitation of PMCA2 protein normalized to beta-actin protein levels in blots from C and D. Protein levels generally paralleled mRNA levels, with the exception that PMCA2 mRNA was detectable in dfw-2J/dfw-2J mice, but PMCA2 protein was not. An asterisk denotes statistical significance.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
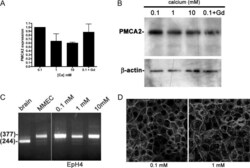
- Fig. 6. A, PMCA2 mRNA expression measured by QRT-PCR in EpH4 mouse MECs exposed to varying levels of extracellular calcium concentrations. Increasing doses of calcium or gadolinium do not increase PMCA2 mRNA expression. B, Western analysis of PMCA2 protein in EpH4 cells incubated overnight in 0.1, 1, or 10 m m calcium or 0.1 m m calcium with 100 m m gadolinium. The calcium concentration and gadolinium treatment had no effect on the amount of PMCA2 protein or its electrophoretic mobility. C, RT-PCR analysis of PMCA2 splice site A in brain (lane 1), primary mouse MECs (MMEC, lane 2), and EpH4 cells incubated in media containing 0.1 (lane 3), 1 (lane 4), or 10 (lane 5) m m calcium reveals that the PMCA2w variant is expressed in MECs, regardless of the calcium concentration. The samples were all run on the same gel, and the space between lanes 2 and 3 is where intervening lanes were removed from the image. D, Immunofluorescent staining for PMCA2 in EpH4 cells incubated for 3 h in media containing 0.1 or 1 m m calcium. The pattern of PMCA2 staining is consistent with plasma membrane localization and is unchanged at 0.1 vs . 1 m m extracellular calcium.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
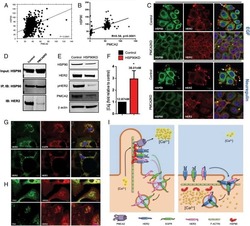
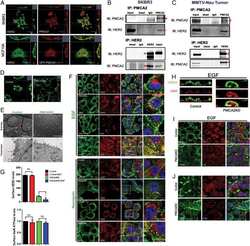
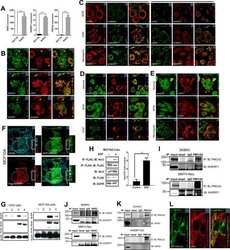
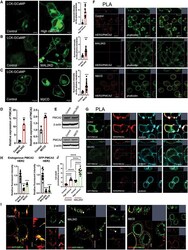
- Figure 5. MAL2 and lipid rafts maintain interactions between HER2 and PMCA2 required to maintain low calcium concentrations at the plasma membrane (A) Live cell confocal images in SKBR3 cells expressing LCK-GCaMP at physiologic calcium (control) or treated with 10 mM extracellular calcium + 10 muM ionomycin. (B) Live cell confocal images in control and MAL2KD_SKBR3 cells expressing LCK-GCaMP. (C) Live cell confocal images in control and MbetaCD-treated SKBR3 cells expressing LCK-GCaM. (D) PMCA2 mRNA expression in control, MAL2KD-treated, or MbetaCD-treated SKBR3 cells as assessed by quantitative RT-PCR (n = 3). (E) Western blot analysis of PMCA2 protein levels in control, MAL2KD-treated, or MbetaCD-treated SKBR3 cells. (F) PLA with immunofluorescence for phalloidin in HER2 and PMCA2 in control (top row), MAL2KD-treated (middle row), and MbetaCD-treated (bottom row) SKBR3 cells. Scale bars represent 10 mum. (G) PLA for HER2 and GFP-PMCA2 with immunofluorescence for GFP-labeled PMCA2 and actin (phalloidin) in control (top row), MAL2KD-treated (middle row), and MbetaCD-treated (bottom row) SKBR3 cells. (H) Quantitation of PLA experiment from (F) and (G) by measuring the fluorescent intensity of amplified PLA reactions at the plasma membrane. (I) SIM imaging showing HER2- and GFP-labeled PMCA2 in control, MAL2KD-treated, and MbetaCD-treated SKBR3 cells. Scale bars represent 10 mum. (J) Apoptosis, as assessed by TUNEL assay, in MAL2KD cells relative to controls exposed to 5 mM cal
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
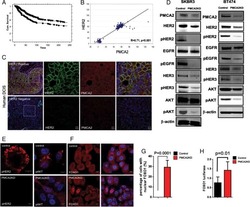
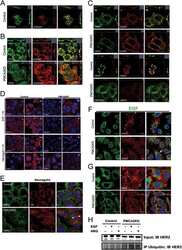
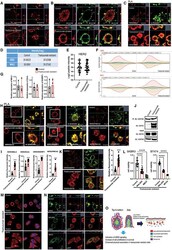
- Figure 6. Enhanced interactions between HER2 and MAL2, Ezrin, NHERF1, and PMCA2 in trastuzumab-resistant SKBR3 cells (A) Immunofluorescence staining for cholera toxin B (lipid rafts) in control and trastuzumab-resistant SKBR3 cells. Scale bars represent 10 mum. (B) Immunofluorescence staining for HER2 and MAL2 in control and trastuzumab-resistant SKBR3 cells. Scale bars represent 10 mum. (C) PLA for HER2 and MAL2 in control and trastuzumab-resistant SKBR3 cells also stained for phalloidin. Scale bars represent 10 mum. (D-F) Quantitative results from immunoprecipitation coupled with data-independent acquisition mass spectrometry (DIA-MS) in control and trastuzumab-resistant SKBR3 cells. (D) The DIA-MS Intensity (log 2 ) of HER2 and MAL2 proteins from control and trastuzumab-resistant SKBR3 cells. (E) The DIA-MS Intensity (log 2 ) for all the peptide precursor signals of HER2 in control and resistant cells. (F) The DIA-MS peak groups visualized for quantifying HER2 (VLGSGAFGTVYK) and MAL2 (VTLPAGPDILR). Peaks above and below the middle line denote the MS2 and MS1 ion traces in DIA-MS. (G) MAL2, Ezrin, and NHERF1 mRNA expression in control and trastuzumab-resistant SKBR3 cells as assessed by quantitative RT-PCR (n = 3). (H) PLA for HER2 with Ezrin (left), NHERF1 (middle), and PMCA2 (right) in control and trastuzumab-resistant SKBR3 cells also stained for phalloidin. Boxed portions are amplified at right with co-registration of PLA signal and immunofluorescence for actin (phalloi
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
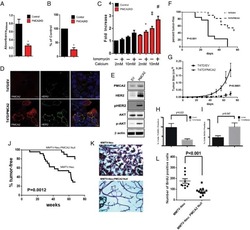
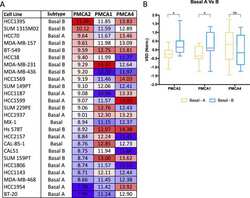
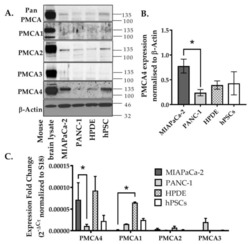
- Figure 2 Expression of PMCA isoforms in multiple pancreatic cell lines. ( A ) Representative Western immunoblot showing the relative protein expression of total/pan-PMCA and PMCA isoform 1-4 in pancreatic cancer (MIA PaCa-2 and PANC-1) and non-malignant pancreatic cells (human pancreatic ductal epithelial (HPDE) and human pancreatic stellate cells (hPSC)). Mouse brain lysate was used as a positive control for PMCA expressions and beta-Actin was used as a protein loading control. ( B ) PMCA4 protein expression in each cell line was quantified from Western blot bands and normalized to beta-Actin housekeeping protein. ( C ) The relative expressions of ATP2B1-4 (PMCA1-4 mRNA) in each cell line were quantified by RT-qPCR. Data are expressed as relative mRNA expression normalized to corresponding S18 rRNA controls (2 -DeltaCtau ). Statistical comparisons were made using the Kruskal-Wallis test with Dunn's multiple comparison test and two-way analysis of variance (ANOVA) with Dunnett's multiple comparison test. Data are expressed as mean +- SEM. (n = 4-5, 4 replicates per treatment condition). * represents statistical significance where p < 0.05.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry