Antibody data
- Antibody Data
- Antigen structure
- References [9]
- Comments [0]
- Validations
- Other assay [7]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 44-224 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-LAT (Tyr132) Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Reactivity
- Human
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Storage
- -20°C
Submitted references Kinetic proofreading through the multi-step activation of the ZAP70 kinase underlies early T cell ligand discrimination.
PLCγ1 promotes phase separation of T cell signaling components.
The Cish SH2 domain is essential for PLC-γ1 regulation in TCR stimulated CD8(+) T cells.
LFA-1 activates focal adhesion kinases FAK1/PYK2 to generate LAT-GRB2-SKAP1 complexes that terminate T-cell conjugate formation.
Small molecule inhibition of Csk alters affinity recognition by T cells.
Quantitative and temporal requirements revealed for Zap70 catalytic activity during T cell development.
Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling.
Stability of an autoinhibitory interface in the structure of the tyrosine kinase ZAP-70 impacts T cell receptor response.
Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases.
Voisinne G, Locard-Paulet M, Froment C, Maturin E, Menoita MG, Girard L, Mellado V, Burlet-Schiltz O, Malissen B, Gonzalez de Peredo A, Roncagalli R
Nature immunology 2022 Sep;23(9):1355-1364
Nature immunology 2022 Sep;23(9):1355-1364
PLCγ1 promotes phase separation of T cell signaling components.
Zeng L, Palaia I, Šarić A, Su X
The Journal of cell biology 2021 Jun 7;220(6)
The Journal of cell biology 2021 Jun 7;220(6)
The Cish SH2 domain is essential for PLC-γ1 regulation in TCR stimulated CD8(+) T cells.
Guittard G, Dios-Esponera A, Palmer DC, Akpan I, Barr VA, Manna A, Restifo NP, Samelson LE
Scientific reports 2018 Mar 28;8(1):5336
Scientific reports 2018 Mar 28;8(1):5336
LFA-1 activates focal adhesion kinases FAK1/PYK2 to generate LAT-GRB2-SKAP1 complexes that terminate T-cell conjugate formation.
Raab M, Lu Y, Kohler K, Smith X, Strebhardt K, Rudd CE
Nature communications 2017 Jul 12;8:16001
Nature communications 2017 Jul 12;8:16001
Small molecule inhibition of Csk alters affinity recognition by T cells.
Manz BN, Tan YX, Courtney AH, Rutaganira F, Palmer E, Shokat KM, Weiss A
eLife 2015 Aug 24;4
eLife 2015 Aug 24;4
Quantitative and temporal requirements revealed for Zap70 catalytic activity during T cell development.
Au-Yeung BB, Melichar HJ, Ross JO, Cheng DA, Zikherman J, Shokat KM, Robey EA, Weiss A
Nature immunology 2014 Jul;15(7):687-94
Nature immunology 2014 Jul;15(7):687-94
Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling.
Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A
Nature immunology 2014 Feb;15(2):186-94
Nature immunology 2014 Feb;15(2):186-94
Stability of an autoinhibitory interface in the structure of the tyrosine kinase ZAP-70 impacts T cell receptor response.
Deindl S, Kadlecek TA, Cao X, Kuriyan J, Weiss A
Proceedings of the National Academy of Sciences of the United States of America 2009 Dec 8;106(49):20699-704
Proceedings of the National Academy of Sciences of the United States of America 2009 Dec 8;106(49):20699-704
Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases.
Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A
Molecular and cellular biology 2005 Jun;25(12):4924-33
Molecular and cellular biology 2005 Jun;25(12):4924-33
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
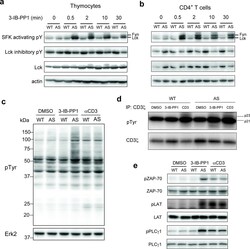
- Figure 1 Inhibition of Csk AS in primary murine T cells induces hyperactivation of SFKs and phosphorylation of TCR proximal signaling molecules. ( a ) Wild-type (WT) or Csk AS (AS) thymocytes or ( b ) peripheral CD4 + T cells were treated for the indicated times with 10 uM 3-IB-PP1 and analyzed by immunoblotting. ( c ) Wild-type (WT) or Csk AS (AS) thymocytes were treated for 3 min with vehicle (DMSO), 3-IB-PP1 or anti-CD3epsilon and analyzed by immunoblotting. ( d ) Wild-type (WT) or Csk AS (AS) thymocytes were treated for 3 min with vehicle (DMSO), 10uM 3-IB-PP1 or 20ug/mL anti-CD3epsilon, then lysed and immunoprecipitated with anti-CD3 zeta antibody. Immunoprecipitates were analyzed by immunoblotting. ( e ) Wild-type (WT) or Csk AS (AS) thymocytes were treated for 3 min with vehicle (DMSO), 10uM 3-IB-PP1 or 20ug/mL anti-CD3epsilon and analyzed by immunoblotting. All data are representative of 3 independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
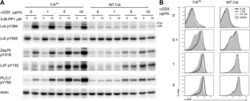
- Figure 1. Inhibiting Csk increases the magnitude of ligand-induced TCR signaling and reduces the threshold for TCR activation. ( A ) Purified Csk AS (AS) or wildtype (WT) CD4 + T cells stimulated for 2 min with 1 mug/ml, 5 mug/ml or 10 mug/ml anti-CD3epsilon antibody in the presence of DMSO or 10 muM 3-IB-PP1 were analyzed by immunoblotting for the phosphorylation of the activation loop tyrosine of Lck and Fyn (Src pY416 antibody) and the inhibitory tyrosine of Lck (Lck pY505), phosphorylated ZAP-70 (ZAP70 pY319), LAT (LAT pY132) and PLC-gamma1 (PLCgamma1 pY783), as well as total actin (loading control). Data are representative of at least three independent experiments. ( B ) Purified total Csk AS (AS) or wildtype (WT) T cells stimulated with the indicated dose of anti-CD3epsilon antibody for 2 min in the presence of DMSO or 5 muM, 1 muM or 0.4 muM 3-IB-PP1 were analyzed for phosphorylated ERK (p-ERK) by phosphoflow. Histograms were gated on CD4 + cells. Data are representative of at least three independent experiments for AS cells and two independent experiments for WT cells. DOI: http://dx.doi.org/ Figure 1--figure supplement 1. Inhibiting Csk during TCR stimulation prolongs TCR signals. ( A ) Purified Csk AS CD4 + T cells stimulated for 2, 5 or 10 min with anti-CD3epsilon antibody in the presence of DMSO or 10 muM 3-IB-PP1 were analyzed by immunoblotting for the phosphorylated ZAP-70, LAT and PLC-gamma1 as well as total GAPDH (loading control). Data are representative of a
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
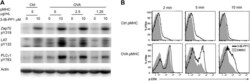
- Figure 2. Inhibiting Csk reduces the threshold for TCR activation and prolongs signaling induced by pMHC engagement. ( A ) Purified Csk AS ;OTII CD4 + T cells stimulated for 3 min with 5 mug/ml bead-bound control pMHC tetramer or 5 mug/ml, 2.5 mug/ml or 1.25 mug/ml bead-bound OVA pMHC tetramer in the presence of DMSO or 10 muM 3-IB-PP1 were analyzed by immunoblotting for the phosphorylated ZAP-70, LAT and PLC-gamma1, as well as total actin (loading control). Data are representative of three independent experiments. ( B ) Purified Csk AS ;OTII CD4 + T cells stimulated with 5 mug/ml bead-bound control-pMHC tetramer or 2.5 mug/ml bead-bound OVA pMHC tetramer for 2, 5 or 10 min in the presence of DMSO or 5 muM 3-IB-PP1 were analyzed for phosphorylated ERK (p-ERK) by phosphoflow. Histograms were gated on CD4 + Valpha2 + cells. Data are representative of three independent experiments. DOI: http://dx.doi.org/ Figure 2--figure supplement 1. Csk inhibition shifts the threshold for cellular proliferation in response to anti-CD3 or p-MHC tetramer stimulation. ( A ) Purified CD4 + T cells from Csk AS mice were loaded with CFSE and stimulated with 3 mug/ml, 1 mug/ml or 0.3 mug/ml plate-bound anti-CD3epsilon and 1 mug/ml anti-CD28 for 72 hr in the presence or absence of 5 muM 3-IB-PP1. Data are representative of three independent experiments. ( B ) Purified CD4 + T cells from Csk AS ;OT-II mice were loaded with CFSE and stimulated with 0.3 mug/ml, 0.1 mug/ml or 0.04 mug/ml plate-bound OVA
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
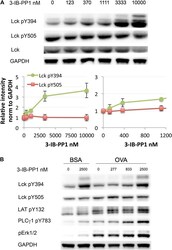
- Figure 3. Weak Csk inhibition and Lck activation potentiate agonist signaling. ( A ) Csk AS CD8 + T cells were rested and stimulated with indicated doses of 3-IB-PP1 for 3 min, lysed, and immunoblotted for activating (pY394) and inhibitory (pY505) site Lck phosphorylation. Below is quantification (+- sem) of three independent experiments immunoblot. ( B ) Csk AS ;OTI naive CD8 + T cells were rested, stimulated with bead-bound BSA or 10 mug/ml pMHC-OVA for 3 min, lysed and assayed by immunoblot. Data are representative of at least two independent experiments. DOI: http://dx.doi.org/ Figure 3--figure supplement 1. Comparison of Csk inhibition and Lck activation in CD4 + vs CD8 + T cells. ( A ) Csk AS CD4 + and Csk AS CD8 + T cells were rested and stimulated with indicated doses of 3-IB-PP1 or DMSO for 2 min and then lysed. Lysates were immunoblotted for activating (pY394) and inhibitory (pY505) site Lck phosphorylation, total Lck, and GAPDH (loading control). Data are representative of three independent experiments. ( B , C ) Quantification of Lck pY505 and Lck pY394 of three independent experiments (as in A ), with levels normalized to Lck levels per cell type. Error bars are standard deviation. DOI: http://dx.doi.org/
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
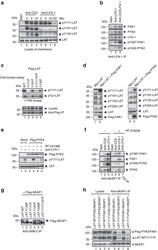
- Figure 4 LFA-1, FAK1 and PYK2 phosphorylate restricted site Y-171 on LAT. ( a ) Anti-LFA-1 cross-linking selectively phosphorylates LAT Y-171. Jurkat cells were ligated with anti-CD3 or anti-LFA-1/CD3 for 2-20 min, followed by membrane preparation and blotting for pY-171-LAT, pY-191-LAT, pY-132-LAT and with anti-LAT ( n =3). ( b ) LFA-1 associates with and activates FAK1 and PYK2. Jurkat cells were ligated with anti-CD3 and/or anti-LFA-1 prior to anti-LFA-1 precipitation and blotting with anti-LFA-1, anti-pY-397-FAK, anti-pY-402-PYK2, anti-FAK1 and anti-PYK2 ( n =3). ( c ) FAK1 in vitro kinase phosphorylation of LAT is dependent on the Y-171 residue. 293T cells were transfected with Flag-tagged LAT-mutants, precipitated with anti-Flag and subjected to a cold in vitro kinase assay with recombinant FAK kinase (Millipore), followed by blotting with ant-pY-171-LAT, anti-pTyr (4610) and anti-Flag ( n =3). ( d ) Co-expression of LAT with FAK1 or PYK2 selectively phosphorylates Y-171. 293T cells were transfected with Myc-tagged LAT and Flag-tagged FAK1 or PYK2 and subjected to blotting with anti-phospho-specific antibodies against LAT. Left panel: Myc-LAT and Flag-FAK1; right panel: Myc-LAT and Flag-PYK2 ( n =3). ( e ) Anti-LFA-1 induced pY-171 is inhibited by FAK1/PYK2 inhibitor PF 431396. Jurkat T-cells transfected with either vector control (mock) or Flag-PYK2 were ligated with anti-LFA-1 (1 mug ml -1 ) in the absence or presence of FAK1/PYK2 inhibitor PF 431396, followed by blot
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
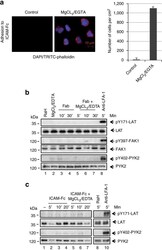
- Figure 5 LFA-1 cross-linking needed for pY-171 and FAK auto-phosphorylation. ( a ) MgCl 2 /EDTA induced the adhesion of T cells to ICAM-1 on plates. Left panels: images of T cells bound to ICAM-Fc (left: untreated controls; right: MgCl 2 /EDTA treated cells stained with DAP1 and TRITC-phalloidin). Right panel: histogram showing MgCl 2 /EDTA induced increases in T-cell binding to ICAM1-Fc ( n =3). ( b ) Antibody cross-linking is needed to induce pY-171 and FAK/PYK2 auto-phosphorylation. Jurkat cells were either incubated with soluble anti-LFA-1 Fab' or Fab' plus MgCl 2 /EDTA or full sized anti-LFA-1 for various times followed by blotting with anti-pY171- LAT, anti-LAT, anti-pY-397-FAK, anti-FAK or anti-pY-402-PYK2 and anti-PYK2. Assay was performed in the absence of ICAM1 on plates ( n =3). ( c ) Mono-valent ICAM1 failed to induce pY-171 and FAK/PYK2 auto-phosphorylation. Jurkat cells were either incubated with soluble ICAM1-Fc or ICAM1-Fc plus MgCl 2 /EDTA or bivalent anti-LFA-1 for various times followed by blotting with anti-pY171- LAT, anti-LAT, anti-pY-402 PYK2 and anti-PYK2. Assay was performed in the absence of ICAM1 on plates ( n =3).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Other assay
Other assay