Antibody data
- Antibody Data
- Antigen structure
- References [105]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [4]
- Other assay [17]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-911 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- SERCA1 ATPase Monoclonal Antibody (IIH11)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA3-911 detects sarcoplasmic or endoplasmic reticulum calcium 1 (SERCA1) ATPase from canine, human, rabbit, rat, mouse and guinea pig tissues. MA3-911 has been successfully used in Western blot, immunohistochemistry, and immunofluorescence procedures. By Western blot, this antibody detects an ~110 kDa protein representing SERCA1 ATPase in canine skeletal muscle extracts. Immunofluorescence staining of SERCA1 ATPase in canine skeletal muscle with MA3-911 results in strong labeling of the entire type II (fast) myofiber. The MA3-911 antigen is purified rabbit skeletal muscle sarcoplasmic reticulum. This antibody recognizes an epitope between amino acids 199-505 of rabbit skeletal muscle ATPase, a region that is not exposed in native sarcoplasmic reticulum.
- Reactivity
- Human, Mouse, Rat, Canine, Guinea Pig, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- IIH11
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Neuronatin promotes SERCA uncoupling and its expression is altered in skeletal muscles of high-fat diet-fed mice.
A chronic high-fat diet exacerbates contractile dysfunction with impaired intracellular Ca(2+) release capacity in the skeletal muscle of aged mice.
Nebulin nemaline myopathy recapitulated in a compound heterozygous mouse model with both a missense and a nonsense mutation in Neb.
Sex differences in mitochondrial Ca(2+) handling in mouse fast-twitch skeletal muscle in vivo.
Electrical Stimulation Prevents Preferential Skeletal Muscle Myosin Loss in Steroid-Denervation Rats.
Myostatin inhibition using mRK35 produces skeletal muscle growth and tubular aggregate formation in wild type and TgACTA1D286G nemaline myopathy mice.
Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension.
A chemical chaperone improves muscle function in mice with a RyR1 mutation.
Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle.
Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding.
Ca(2+) permeation and/or binding to CaV1.1 fine-tunes skeletal muscle Ca(2+) signaling to sustain muscle function.
Muscle dysfunction associated with adjuvant-induced arthritis is prevented by antioxidant treatment.
Identification of Small Ankyrin 1 as a Novel Sarco(endo)plasmic Reticulum Ca2+-ATPase 1 (SERCA1) Regulatory Protein in Skeletal Muscle.
Expression of calcium-buffering proteins in rat intrinsic laryngeal muscles.
Mitsugumin 56 (hedgehog acyltransferase-like) is a sarcoplasmic reticulum-resident protein essential for postnatal muscle maturation.
Higher PLIN5 but not PLIN3 content in isolated skeletal muscle mitochondria following acute in vivo contraction in rat hindlimb.
Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice.
Myoplasmic resting Ca2+ regulation by ryanodine receptors is under the control of a novel Ca2+-binding region of the receptor.
Vesicular transport system in myotubes: ultrastructural study and signposting with vesicle-associated membrane proteins.
In vivo imaging of intracellular Ca2+ after muscle contractions and direct Ca2+ injection in rat skeletal muscle in diabetes.
Adaptations of mouse skeletal muscle to low-intensity vibration training.
Diaphragm muscle remodeling in a rat model of chronic intermittent hypoxia.
The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy.
Primary over-expression of AβPP in muscle does not lead to the development of inclusion body myositis in a new lineage of the MCK-AβPP transgenic mouse.
Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells.
Isolation of sarcolemmal plasma membranes by mechanically skinning rat skeletal muscle fibers for phospholipid analysis.
Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle in vivo.
Phosphatase-dead myotubularin ameliorates X-linked centronuclear myopathy phenotypes in mice.
Regulation and rate limiting mechanisms of Ca2+ ATPase (SERCA2) expression in cardiac myocytes.
Exposure to low mercury concentration in vivo impairs myocardial contractile function.
Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle.
Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies.
Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion.
Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression.
Superior calcium homeostasis of extraocular muscles.
Calsequestrin and junctin immunoreactivity in hexagonally cross-linked tubular arrays myopathy.
Fusion of the endoplasmic reticulum and mitochondrial outer membrane in rats brown adipose tissue: activation of thermogenesis by Ca2+.
Succinate modulates Ca(2+) transient and cardiomyocyte viability through PKA-dependent pathway.
Stable structural analog of Ca2+-ATPase ADP-insensitive phosphoenzyme with occluded Ca2+ formed by elongation of A-domain/M1'-linker and beryllium fluoride binding.
Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice.
T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase.
Sarcoplasmic-endoplasmic-reticulum Ca2+-ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice.
AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis.
Conformational fluctuations of the Ca2+-ATPase in the native membrane environment. Effects of pH, temperature, catalytic substrates, and thapsigargin.
Effects of high-affinity inhibitors on partial reactions, charge movements, and conformational States of the Ca2+ transport ATPase (sarco-endoplasmic reticulum Ca2+ ATPase).
SRP-27 is a novel component of the supramolecular signalling complex involved in skeletal muscle excitation-contraction coupling.
Proteomic profiling of chronic low-frequency stimulated fast muscle.
Cellular trafficking of phospholamban and formation of functional sarcoplasmic reticulum during myocyte differentiation.
Thermogenic activity of Ca2+-ATPase from skeletal muscle heavy sarcoplasmic reticulum: the role of ryanodine Ca2+ channel.
Sarco(endo)plasmic reticulum calcium pump isoforms in paralyzed rat slow muscle.
UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice.
Identification of a Ca2+-ATPase in brown adipose tissue mitochondria: regulation of thermogenesis by ATP and Ca2+.
Differential expression of the fast skeletal muscle proteome following chronic low-frequency stimulation.
Expression of the skeletal muscle dystrophin-dystroglycan complex and syntrophin-nitric oxide synthase complex is severely affected in the type 2 diabetic Goto-Kakizaki rat.
Interference with phosphoenzyme isomerization and inhibition of the sarco-endoplasmic reticulum Ca2+ ATPase by 1,3-dibromo-2,4,6-tris(methylisothiouronium) benzene.
Expression levels of RyR1 and RyR3 control resting free Ca2+ in skeletal muscle.
The thermogenic activity of rat brown adipose tissue and rabbit white muscle Ca2+-ATPase.
Subproteomics analysis of Ca+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle.
Skeletal muscle sarcoplasmic reticulum glycogen status influences Ca2+ uptake supported by endogenously synthesized ATP.
Drastic reduction of sarcalumenin in Dp427 (dystrophin of 427 kDa)-deficient fibres indicates that abnormal calcium handling plays a key role in muscular dystrophy.
Myosin heavy chain and physiological adaptation of the rat diaphragm in elastase-induced emphysema.
Myosin heavy chain and physiological adaptation of the rat diaphragm in elastase-induced emphysema.
Brown adipose tissue Ca2+-ATPase: uncoupled ATP hydrolysis and thermogenic activity.
Hyperthyroidism increases the uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2+-ATPase.
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Mechanisms underlying increases in SR Ca2+-ATPase activity after exercise in rat skeletal muscle.
Increased expression of the nicotinic acetylcholine receptor in stimulated muscle.
Increased expression of the nicotinic acetylcholine receptor in stimulated muscle.
Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA1 and -2) in human extraocular muscles.
Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA1 and -2) in human extraocular muscles.
Use of continuous-elution gel electrophoresis as a preparative tool for blot overlay analysis.
Supramolecular calsequestrin complex.
Supramolecular calsequestrin complex.
TGF-beta-induced Ca(2+) influx involves the type III IP(3) receptor and regulates actin cytoskeleton.
Effects of moderate heart failure and functional overload on rat plantaris muscle.
Effects of moderate heart failure and functional overload on rat plantaris muscle.
Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle.
Identification of Ank(G107), a muscle-specific ankyrin-G isoform.
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Identification of a 97-kDa mastoparan-binding protein involving in Ca(2+) release from skeletal muscle sarcoplasmic reticulum.
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Two isoforms of sarco/endoplasmic reticulum calcium ATPase (SERCA) are essential in Caenorhabditis elegans.
Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle.
Type 3 and type 1 ryanodine receptors are localized in triads of the same mammalian skeletal muscle fibers.
Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function.
Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function.
Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle.
Transcriptional regulation of phospholamban gene and translational regulation of SERCA2 gene produces coordinate expression of these two sarcoplasmic reticulum proteins during skeletal muscle phenotype switching.
Transcriptional regulation of phospholamban gene and translational regulation of SERCA2 gene produces coordinate expression of these two sarcoplasmic reticulum proteins during skeletal muscle phenotype switching.
Characterization and ultrastructural localization of a novel 90-kDa protein unique to skeletal muscle junctional sarcoplasmic reticulum.
Biochemical characterization of ultrastructural localization of a major junctional sarcoplasmic reticulum glycoprotein (triadin).
Immunological relatedness of the sarcoplasmic reticulum Ca(2+)-ATPase and the Na+,K(+)-ATPase.
Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma.
Analysis of excitation-contraction-coupling components in chronically stimulated canine skeletal muscle.
Analysis of excitation-contraction-coupling components in chronically stimulated canine skeletal muscle.
Ca-ATPase isozyme expression in sarcoplasmic reticulum is altered by chronic stimulation of skeletal muscle.
The binding of monoclonal and polyclonal antibodies to the Ca2(+)-ATPase of sarcoplasmic reticulum: effects on interactions between ATPase molecules.
A monoclonal antibody to the Ca2+-ATPase of cardiac sarcoplasmic reticulum cross-reacts with slow type I but not with fast type II canine skeletal muscle fibers: an immunocytochemical and immunochemical study.
Braun JL, Teng ACT, Geromella MS, Ryan CR, Fenech RK, MacPherson REK, Gramolini AO, Fajardo VA
FEBS letters 2021 Nov;595(22):2756-2767
FEBS letters 2021 Nov;595(22):2756-2767
A chronic high-fat diet exacerbates contractile dysfunction with impaired intracellular Ca(2+) release capacity in the skeletal muscle of aged mice.
Eshima H, Tamura Y, Kakehi S, Kakigi R, Hashimoto R, Funai K, Kawamori R, Watada H
Journal of applied physiology (Bethesda, Md. : 1985) 2020 May 1;128(5):1153-1162
Journal of applied physiology (Bethesda, Md. : 1985) 2020 May 1;128(5):1153-1162
Nebulin nemaline myopathy recapitulated in a compound heterozygous mouse model with both a missense and a nonsense mutation in Neb.
Laitila JM, McNamara EL, Wingate CD, Goullee H, Ross JA, Taylor RL, van der Pijl R, Griffiths LM, Harries R, Ravenscroft G, Clayton JS, Sewry C, Lawlor MW, Ottenheijm CAC, Bakker AJ, Ochala J, Laing NG, Wallgren-Pettersson C, Pelin K, Nowak KJ
Acta neuropathologica communications 2020 Feb 17;8(1):18
Acta neuropathologica communications 2020 Feb 17;8(1):18
Sex differences in mitochondrial Ca(2+) handling in mouse fast-twitch skeletal muscle in vivo.
Watanabe D, Hatakeyama K, Ikegami R, Eshima H, Yagishita K, Poole DC, Kano Y
Journal of applied physiology (Bethesda, Md. : 1985) 2020 Feb 1;128(2):241-251
Journal of applied physiology (Bethesda, Md. : 1985) 2020 Feb 1;128(2):241-251
Electrical Stimulation Prevents Preferential Skeletal Muscle Myosin Loss in Steroid-Denervation Rats.
Yamada T, Himori K, Tatebayashi D, Yamada R, Ashida Y, Imai T, Akatsuka M, Masuda Y, Kanzaki K, Watanabe D, Wada M, Westerblad H, Lanner JT
Frontiers in physiology 2018;9:1111
Frontiers in physiology 2018;9:1111
Myostatin inhibition using mRK35 produces skeletal muscle growth and tubular aggregate formation in wild type and TgACTA1D286G nemaline myopathy mice.
Tinklenberg JA, Siebers EM, Beatka MJ, Meng H, Yang L, Zhang Z, Ross JA, Ochala J, Morris C, Owens JM, Laing NG, Nowak KJ, Lawlor MW
Human molecular genetics 2018 Feb 15;27(4):638-648
Human molecular genetics 2018 Feb 15;27(4):638-648
Superoxide dismutase/catalase mimetic EUK-134 prevents diaphragm muscle weakness in monocrotalin-induced pulmonary hypertension.
Himori K, Abe M, Tatebayashi D, Lee J, Westerblad H, Lanner JT, Yamada T
PloS one 2017;12(2):e0169146
PloS one 2017;12(2):e0169146
A chemical chaperone improves muscle function in mice with a RyR1 mutation.
Lee CS, Hanna AD, Wang H, Dagnino-Acosta A, Joshi AD, Knoblauch M, Xia Y, Georgiou DK, Xu J, Long C, Amano H, Reynolds C, Dong K, Martin JC, Lagor WR, Rodney GG, Sahin E, Sewry C, Hamilton SL
Nature communications 2017 Mar 24;8:14659
Nature communications 2017 Mar 24;8:14659
Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle.
Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K, Kakigi R, Okada T, Sakurai T, Kawamori R, Watada H
Physiological reports 2017 Apr;5(7)
Physiological reports 2017 Apr;5(7)
Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding.
De Andrade PB, Neff LA, Strosova MK, Arsenijevic D, Patthey-Vuadens O, Scapozza L, Montani JP, Ruegg UT, Dulloo AG, Dorchies OM
Frontiers in physiology 2015;6:254
Frontiers in physiology 2015;6:254
Ca(2+) permeation and/or binding to CaV1.1 fine-tunes skeletal muscle Ca(2+) signaling to sustain muscle function.
Lee CS, Dagnino-Acosta A, Yarotskyy V, Hanna A, Lyfenko A, Knoblauch M, Georgiou DK, Poché RA, Swank MW, Long C, Ismailov II, Lanner J, Tran T, Dong K, Rodney GG, Dickinson ME, Beeton C, Zhang P, Dirksen RT, Hamilton SL
Skeletal muscle 2015;5:4
Skeletal muscle 2015;5:4
Muscle dysfunction associated with adjuvant-induced arthritis is prevented by antioxidant treatment.
Yamada T, Abe M, Lee J, Tatebayashi D, Himori K, Kanzaki K, Wada M, Bruton JD, Westerblad H, Lanner JT
Skeletal muscle 2015;5:20
Skeletal muscle 2015;5:20
Identification of Small Ankyrin 1 as a Novel Sarco(endo)plasmic Reticulum Ca2+-ATPase 1 (SERCA1) Regulatory Protein in Skeletal Muscle.
Desmond PF, Muriel J, Markwardt ML, Rizzo MA, Bloch RJ
The Journal of biological chemistry 2015 Nov 13;290(46):27854-67
The Journal of biological chemistry 2015 Nov 13;290(46):27854-67
Expression of calcium-buffering proteins in rat intrinsic laryngeal muscles.
Ferretti R, Marques MJ, Khurana TS, Santo Neto H
Physiological reports 2015 Jun;3(6)
Physiological reports 2015 Jun;3(6)
Mitsugumin 56 (hedgehog acyltransferase-like) is a sarcoplasmic reticulum-resident protein essential for postnatal muscle maturation.
Van B, Nishi M, Komazaki S, Ichimura A, Kakizawa S, Nakanaga K, Aoki J, Park KH, Ma J, Ueyama T, Ogata T, Maruyama N, Takeshima H
FEBS letters 2015 Apr 28;589(10):1095-104
FEBS letters 2015 Apr 28;589(10):1095-104
Higher PLIN5 but not PLIN3 content in isolated skeletal muscle mitochondria following acute in vivo contraction in rat hindlimb.
Ramos SV, MacPherson RE, Turnbull PC, Bott KN, LeBlanc P, Ward WE, Peters SJ
Physiological reports 2014 Oct 1;2(10)
Physiological reports 2014 Oct 1;2(10)
Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice.
Burr AR, Millay DP, Goonasekera SA, Park KH, Sargent MA, Collins J, Altamirano F, Philipson KD, Allen PD, Ma J, López JR, Molkentin JD
Molecular and cellular biology 2014 Jun;34(11):1991-2002
Molecular and cellular biology 2014 Jun;34(11):1991-2002
Myoplasmic resting Ca2+ regulation by ryanodine receptors is under the control of a novel Ca2+-binding region of the receptor.
Chen Y, Xue S, Zou J, Lopez JR, Yang JJ, Perez CF
The Biochemical journal 2014 Jun 1;460(2):261-71
The Biochemical journal 2014 Jun 1;460(2):261-71
Vesicular transport system in myotubes: ultrastructural study and signposting with vesicle-associated membrane proteins.
Tajika Y, Takahashi M, Khairani AF, Ueno H, Murakami T, Yorifuji H
Histochemistry and cell biology 2014 Apr;141(4):441-54
Histochemistry and cell biology 2014 Apr;141(4):441-54
In vivo imaging of intracellular Ca2+ after muscle contractions and direct Ca2+ injection in rat skeletal muscle in diabetes.
Eshima H, Tanaka Y, Sonobe T, Inagaki T, Nakajima T, Poole DC, Kano Y
American journal of physiology. Regulatory, integrative and comparative physiology 2013 Sep 15;305(6):R610-8
American journal of physiology. Regulatory, integrative and comparative physiology 2013 Sep 15;305(6):R610-8
Adaptations of mouse skeletal muscle to low-intensity vibration training.
McKeehen JN, Novotny SA, Baltgalvis KA, Call JA, Nuckley DJ, Lowe DA
Medicine and science in sports and exercise 2013 Jun;45(6):1051-9
Medicine and science in sports and exercise 2013 Jun;45(6):1051-9
Diaphragm muscle remodeling in a rat model of chronic intermittent hypoxia.
Shortt CM, Fredsted A, Bradford A, O'Halloran KD
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2013 Jul;61(7):487-99
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2013 Jul;61(7):487-99
The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy.
Dorchies OM, Reutenauer-Patte J, Dahmane E, Ismail HM, Petermann O, Patthey- Vuadens O, Comyn SA, Gayi E, Piacenza T, Handa RJ, Décosterd LA, Ruegg UT
The American journal of pathology 2013 Feb;182(2):485-504
The American journal of pathology 2013 Feb;182(2):485-504
Primary over-expression of AβPP in muscle does not lead to the development of inclusion body myositis in a new lineage of the MCK-AβPP transgenic mouse.
Luo YB, Johnsen RD, Griffiths L, Needham M, Fabian VA, Fletcher S, Wilton SD, Mastaglia FL
International journal of experimental pathology 2013 Dec;94(6):418-25
International journal of experimental pathology 2013 Dec;94(6):418-25
Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells.
Kiyonaka S, Kajimoto T, Sakaguchi R, Shinmi D, Omatsu-Kanbe M, Matsuura H, Imamura H, Yoshizaki T, Hamachi I, Morii T, Mori Y
Nature methods 2013 Dec;10(12):1232-8
Nature methods 2013 Dec;10(12):1232-8
Isolation of sarcolemmal plasma membranes by mechanically skinning rat skeletal muscle fibers for phospholipid analysis.
Fajardo VA, McMeekin L, Basic A, Lamb GD, Murphy RM, LeBlanc PJ
Lipids 2013 Apr;48(4):421-30
Lipids 2013 Apr;48(4):421-30
Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle in vivo.
Amoasii L, Hnia K, Chicanne G, Brech A, Cowling BS, Müller MM, Schwab Y, Koebel P, Ferry A, Payrastre B, Laporte J
Journal of cell science 2013 Apr 15;126(Pt 8):1806-19
Journal of cell science 2013 Apr 15;126(Pt 8):1806-19
Phosphatase-dead myotubularin ameliorates X-linked centronuclear myopathy phenotypes in mice.
Amoasii L, Bertazzi DL, Tronchère H, Hnia K, Chicanne G, Rinaldi B, Cowling BS, Ferry A, Klaholz B, Payrastre B, Laporte J, Friant S
PLoS genetics 2012;8(10):e1002965
PLoS genetics 2012;8(10):e1002965
Regulation and rate limiting mechanisms of Ca2+ ATPase (SERCA2) expression in cardiac myocytes.
Prasad AM, Inesi G
Molecular and cellular biochemistry 2012 Feb;361(1-2):85-96
Molecular and cellular biochemistry 2012 Feb;361(1-2):85-96
Exposure to low mercury concentration in vivo impairs myocardial contractile function.
Furieri LB, Fioresi M, Junior RF, Bartolomé MV, Fernandes AA, Cachofeiro V, Lahera V, Salaices M, Stefanon I, Vassallo DV
Toxicology and applied pharmacology 2011 Sep 1;255(2):193-9
Toxicology and applied pharmacology 2011 Sep 1;255(2):193-9
Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle.
Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD
The Journal of clinical investigation 2011 Mar;121(3):1044-52
The Journal of clinical investigation 2011 Mar;121(3):1044-52
Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies.
Toussaint A, Cowling BS, Hnia K, Mohr M, Oldfors A, Schwab Y, Yis U, Maisonobe T, Stojkovic T, Wallgren-Pettersson C, Laugel V, Echaniz-Laguna A, Mandel JL, Nishino I, Laporte J
Acta neuropathologica 2011 Feb;121(2):253-66
Acta neuropathologica 2011 Feb;121(2):253-66
Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion.
Arruda AP, Milanski M, Coope A, Torsoni AS, Ropelle E, Carvalho DP, Carvalheira JB, Velloso LA
Endocrinology 2011 Apr;152(4):1314-26
Endocrinology 2011 Apr;152(4):1314-26
Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression.
Zhao X, Min CK, Ko JK, Parness J, Kim DH, Weisleder N, Ma J
Biophysical journal 2010 Sep 8;99(5):1556-64
Biophysical journal 2010 Sep 8;99(5):1556-64
Superior calcium homeostasis of extraocular muscles.
Zeiger U, Mitchell CH, Khurana TS
Experimental eye research 2010 Nov;91(5):613-22
Experimental eye research 2010 Nov;91(5):613-22
Calsequestrin and junctin immunoreactivity in hexagonally cross-linked tubular arrays myopathy.
Di Blasi C, Blasevich F, Bellafiore E, Mottarelli E, Gibertini S, Zanotti S, Saredi S, Mantegazza R, Morandi L, Mora M
Neuromuscular disorders : NMD 2010 May;20(5):326-9
Neuromuscular disorders : NMD 2010 May;20(5):326-9
Fusion of the endoplasmic reticulum and mitochondrial outer membrane in rats brown adipose tissue: activation of thermogenesis by Ca2+.
de Meis L, Ketzer LA, da Costa RM, de Andrade IR, Benchimol M
PloS one 2010 Mar 2;5(3):e9439
PloS one 2010 Mar 2;5(3):e9439
Succinate modulates Ca(2+) transient and cardiomyocyte viability through PKA-dependent pathway.
Aguiar CJ, Andrade VL, Gomes ER, Alves MN, Ladeira MS, Pinheiro AC, Gomes DA, Almeida AP, Goes AM, Resende RR, Guatimosim S, Leite MF
Cell calcium 2010 Jan;47(1):37-46
Cell calcium 2010 Jan;47(1):37-46
Stable structural analog of Ca2+-ATPase ADP-insensitive phosphoenzyme with occluded Ca2+ formed by elongation of A-domain/M1'-linker and beryllium fluoride binding.
Daiho T, Danko S, Yamasaki K, Suzuki H
The Journal of biological chemistry 2010 Aug 6;285(32):24538-47
The Journal of biological chemistry 2010 Aug 6;285(32):24538-47
Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice.
Ferreira JC, Bacurau AV, Bueno CR Jr, Cunha TC, Tanaka LY, Jardim MA, Ramires PR, Brum PC
Experimental biology and medicine (Maywood, N.J.) 2010 Apr;235(4):497-505
Experimental biology and medicine (Maywood, N.J.) 2010 Apr;235(4):497-505
T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase.
Al-Qusairi L, Weiss N, Toussaint A, Berbey C, Messaddeq N, Kretz C, Sanoudou D, Beggs AH, Allard B, Mandel JL, Laporte J, Jacquemond V, Buj-Bello A
Proceedings of the National Academy of Sciences of the United States of America 2009 Nov 3;106(44):18763-8
Proceedings of the National Academy of Sciences of the United States of America 2009 Nov 3;106(44):18763-8
Sarcoplasmic-endoplasmic-reticulum Ca2+-ATPase and calsequestrin are overexpressed in spared intrinsic laryngeal muscles of dystrophin-deficient mdx mice.
Ferretti R, Marques MJ, Pertille A, Santo Neto H
Muscle & nerve 2009 May;39(5):609-15
Muscle & nerve 2009 May;39(5):609-15
AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis.
Buj-Bello A, Fougerousse F, Schwab Y, Messaddeq N, Spehner D, Pierson CR, Durand M, Kretz C, Danos O, Douar AM, Beggs AH, Schultz P, Montus M, Denèfle P, Mandel JL
Human molecular genetics 2008 Jul 15;17(14):2132-43
Human molecular genetics 2008 Jul 15;17(14):2132-43
Conformational fluctuations of the Ca2+-ATPase in the native membrane environment. Effects of pH, temperature, catalytic substrates, and thapsigargin.
Inesi G, Lewis D, Toyoshima C, Hirata A, de Meis L
The Journal of biological chemistry 2008 Jan 11;283(2):1189-96
The Journal of biological chemistry 2008 Jan 11;283(2):1189-96
Effects of high-affinity inhibitors on partial reactions, charge movements, and conformational States of the Ca2+ transport ATPase (sarco-endoplasmic reticulum Ca2+ ATPase).
Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Tal DM, Lewis D, Inesi G
Molecular pharmacology 2008 Apr;73(4):1134-40
Molecular pharmacology 2008 Apr;73(4):1134-40
SRP-27 is a novel component of the supramolecular signalling complex involved in skeletal muscle excitation-contraction coupling.
Bleunven C, Treves S, Jinyu X, Leo E, Ronjat M, De Waard M, Kern G, Flucher BE, Zorzato F
The Biochemical journal 2008 Apr 15;411(2):343-9
The Biochemical journal 2008 Apr 15;411(2):343-9
Proteomic profiling of chronic low-frequency stimulated fast muscle.
Donoghue P, Doran P, Wynne K, Pedersen K, Dunn MJ, Ohlendieck K
Proteomics 2007 Sep;7(18):3417-30
Proteomics 2007 Sep;7(18):3417-30
Cellular trafficking of phospholamban and formation of functional sarcoplasmic reticulum during myocyte differentiation.
Stenoien DL, Knyushko TV, Londono MP, Opresko LK, Mayer MU, Brady ST, Squier TC, Bigelow DJ
American journal of physiology. Cell physiology 2007 Jun;292(6):C2084-94
American journal of physiology. Cell physiology 2007 Jun;292(6):C2084-94
Thermogenic activity of Ca2+-ATPase from skeletal muscle heavy sarcoplasmic reticulum: the role of ryanodine Ca2+ channel.
Arruda AP, Nigro M, Oliveira GM, de Meis L
Biochimica et biophysica acta 2007 Jun;1768(6):1498-505
Biochimica et biophysica acta 2007 Jun;1768(6):1498-505
Sarco(endo)plasmic reticulum calcium pump isoforms in paralyzed rat slow muscle.
Talmadge RJ, Paalani M
Biochimica et biophysica acta 2007 Aug;1770(8):1187-93
Biochimica et biophysica acta 2007 Aug;1770(8):1187-93
UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice.
Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP
The Journal of biological chemistry 2006 Oct 20;281(42):31894-908
The Journal of biological chemistry 2006 Oct 20;281(42):31894-908
Identification of a Ca2+-ATPase in brown adipose tissue mitochondria: regulation of thermogenesis by ATP and Ca2+.
de Meis L, Arruda AP, da Costa RM, Benchimol M
The Journal of biological chemistry 2006 Jun 16;281(24):16384-90
The Journal of biological chemistry 2006 Jun 16;281(24):16384-90
Differential expression of the fast skeletal muscle proteome following chronic low-frequency stimulation.
Donoghue P, Doran P, Dowling P, Ohlendieck K
Biochimica et biophysica acta 2005 Sep 25;1752(2):166-76
Biochimica et biophysica acta 2005 Sep 25;1752(2):166-76
Expression of the skeletal muscle dystrophin-dystroglycan complex and syntrophin-nitric oxide synthase complex is severely affected in the type 2 diabetic Goto-Kakizaki rat.
Mulvey C, Harno E, Keenan A, Ohlendieck K
European journal of cell biology 2005 Nov;84(11):867-83
European journal of cell biology 2005 Nov;84(11):867-83
Interference with phosphoenzyme isomerization and inhibition of the sarco-endoplasmic reticulum Ca2+ ATPase by 1,3-dibromo-2,4,6-tris(methylisothiouronium) benzene.
Hua S, Xu C, Ma H, Inesi G
The Journal of biological chemistry 2005 May 6;280(18):17579-83
The Journal of biological chemistry 2005 May 6;280(18):17579-83
Expression levels of RyR1 and RyR3 control resting free Ca2+ in skeletal muscle.
Perez CF, López JR, Allen PD
American journal of physiology. Cell physiology 2005 Mar;288(3):C640-9
American journal of physiology. Cell physiology 2005 Mar;288(3):C640-9
The thermogenic activity of rat brown adipose tissue and rabbit white muscle Ca2+-ATPase.
de Meis L, Oliveira GM, Arruda AP, Santos R, Costa RM, Benchimol M
IUBMB life 2005 Apr-May;57(4-5):337-45
IUBMB life 2005 Apr-May;57(4-5):337-45
Subproteomics analysis of Ca+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle.
Doran P, Dowling P, Lohan J, McDonnell K, Poetsch S, Ohlendieck K
European journal of biochemistry 2004 Oct;271(19):3943-52
European journal of biochemistry 2004 Oct;271(19):3943-52
Skeletal muscle sarcoplasmic reticulum glycogen status influences Ca2+ uptake supported by endogenously synthesized ATP.
Lees SJ, Williams JH
American journal of physiology. Cell physiology 2004 Jan;286(1):C97-104
American journal of physiology. Cell physiology 2004 Jan;286(1):C97-104
Drastic reduction of sarcalumenin in Dp427 (dystrophin of 427 kDa)-deficient fibres indicates that abnormal calcium handling plays a key role in muscular dystrophy.
Dowling P, Doran P, Ohlendieck K
The Biochemical journal 2004 Apr 15;379(Pt 2):479-88
The Biochemical journal 2004 Apr 15;379(Pt 2):479-88
Myosin heavy chain and physiological adaptation of the rat diaphragm in elastase-induced emphysema.
Kim DK, Zhu J, Kozyak BW, Burkman JM, Rubinstein NA, Lankford EB, Stedman HH, Nguyen T, Levine S, Shrager JB
Respiratory research 2003;4(1):1
Respiratory research 2003;4(1):1
Myosin heavy chain and physiological adaptation of the rat diaphragm in elastase-induced emphysema.
Kim DK, Zhu J, Kozyak BW, Burkman JM, Rubinstein NA, Lankford EB, Stedman HH, Nguyen T, Levine S, Shrager JB
Respiratory research 2003;4:1
Respiratory research 2003;4:1
Brown adipose tissue Ca2+-ATPase: uncoupled ATP hydrolysis and thermogenic activity.
de Meis L
The Journal of biological chemistry 2003 Oct 24;278(43):41856-61
The Journal of biological chemistry 2003 Oct 24;278(43):41856-61
Hyperthyroidism increases the uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2+-ATPase.
Arruda AP, Da-Silva WS, Carvalho DP, De Meis L
The Biochemical journal 2003 Nov 1;375(Pt 3):753-60
The Biochemical journal 2003 Nov 1;375(Pt 3):753-60
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Dowling P, Lohan J, Ohlendieck K
European journal of cell biology 2003 May;82(5):222-30
European journal of cell biology 2003 May;82(5):222-30
Comparative analysis of Dp427-deficient mdx tissues shows that the milder dystrophic phenotype of extraocular and toe muscle fibres is associated with a persistent expression of beta-dystroglycan.
Dowling P, Lohan J, Ohlendieck K
European journal of cell biology 2003 May;82(5):222-30
European journal of cell biology 2003 May;82(5):222-30
Mechanisms underlying increases in SR Ca2+-ATPase activity after exercise in rat skeletal muscle.
Schertzer JD, Green HJ, Duhamel TA, Tupling AR
American journal of physiology. Endocrinology and metabolism 2003 Mar;284(3):E597-610
American journal of physiology. Endocrinology and metabolism 2003 Mar;284(3):E597-610
Increased expression of the nicotinic acetylcholine receptor in stimulated muscle.
O'Reilly C, Pette D, Ohlendieck K
Biochemical and biophysical research communications 2003 Jan 10;300(2):585-91
Biochemical and biophysical research communications 2003 Jan 10;300(2):585-91
Increased expression of the nicotinic acetylcholine receptor in stimulated muscle.
O'Reilly C, Pette D, Ohlendieck K
Biochemical and biophysical research communications 2003 Jan 10;300(2):585-91
Biochemical and biophysical research communications 2003 Jan 10;300(2):585-91
Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA1 and -2) in human extraocular muscles.
Kjellgren D, Ryan M, Ohlendieck K, Thornell LE, Pedrosa-Domellöf F
Investigative ophthalmology & visual science 2003 Dec;44(12):5057-62
Investigative ophthalmology & visual science 2003 Dec;44(12):5057-62
Sarco(endo)plasmic reticulum Ca2+ ATPases (SERCA1 and -2) in human extraocular muscles.
Kjellgren D, Ryan M, Ohlendieck K, Thornell LE, Pedrosa-Domellöf F
Investigative ophthalmology & visual science 2003 Dec;44(12):5057-62
Investigative ophthalmology & visual science 2003 Dec;44(12):5057-62
Use of continuous-elution gel electrophoresis as a preparative tool for blot overlay analysis.
Mulvey C, Ohlendieck K
Analytical biochemistry 2003 Aug 1;319(1):122-30
Analytical biochemistry 2003 Aug 1;319(1):122-30
Supramolecular calsequestrin complex.
Glover L, Quinn S, Ryan M, Pette D, Ohlendieck K
European journal of biochemistry 2002 Sep;269(18):4607-16
European journal of biochemistry 2002 Sep;269(18):4607-16
Supramolecular calsequestrin complex.
Glover L, Quinn S, Ryan M, Pette D, Ohlendieck K
European journal of biochemistry 2002 Sep;269(18):4607-16
European journal of biochemistry 2002 Sep;269(18):4607-16
TGF-beta-induced Ca(2+) influx involves the type III IP(3) receptor and regulates actin cytoskeleton.
McGowan TA, Madesh M, Zhu Y, Wang L, Russo M, Deelman L, Henning R, Joseph S, Hajnoczky G, Sharma K
American journal of physiology. Renal physiology 2002 May;282(5):F910-20
American journal of physiology. Renal physiology 2002 May;282(5):F910-20
Effects of moderate heart failure and functional overload on rat plantaris muscle.
Spangenburg EE, Lees SJ, Otis JS, Musch TI, Talmadge RJ, Williams JH
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Jan;92(1):18-24
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Jan;92(1):18-24
Effects of moderate heart failure and functional overload on rat plantaris muscle.
Spangenburg EE, Lees SJ, Otis JS, Musch TI, Talmadge RJ, Williams JH
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Jan;92(1):18-24
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Jan;92(1):18-24
Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle.
Culligan K, Banville N, Dowling P, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Feb;92(2):435-45
Journal of applied physiology (Bethesda, Md. : 1985) 2002 Feb;92(2):435-45
Identification of Ank(G107), a muscle-specific ankyrin-G isoform.
Gagelin C, Constantin B, Deprette C, Ludosky MA, Recouvreur M, Cartaud J, Cognard C, Raymond G, Kordeli E
The Journal of biological chemistry 2002 Apr 12;277(15):12978-87
The Journal of biological chemistry 2002 Apr 12;277(15):12978-87
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Harmon S, Froemming GR, Leisner E, Pette D, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Low-frequency stimulation of fast muscle affects the abundance of Ca(2+)-ATPase but not its oligomeric status.
Harmon S, Froemming GR, Leisner E, Pette D, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Journal of applied physiology (Bethesda, Md. : 1985) 2001 Jan;90(1):371-9
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Froemming GR, Ohlendieck K
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Native skeletal muscle dihydropyridine receptor exists as a supramolecular triad complex.
Froemming GR, Ohlendieck K
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Cellular and molecular life sciences : CMLS 2001 Feb;58(2):312-20
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Glover L, Culligan K, Cala S, Mulvey C, Ohlendieck K
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Calsequestrin binds to monomeric and complexed forms of key calcium-handling proteins in native sarcoplasmic reticulum membranes from rabbit skeletal muscle.
Glover L, Culligan K, Cala S, Mulvey C, Ohlendieck K
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Biochimica et biophysica acta 2001 Dec 1;1515(2):120-32
Identification of a 97-kDa mastoparan-binding protein involving in Ca(2+) release from skeletal muscle sarcoplasmic reticulum.
Hirata Y, Nakahata N, Ohizumi Y
Molecular pharmacology 2000 Jun;57(6):1235-42
Molecular pharmacology 2000 Jun;57(6):1235-42
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Comparative analysis of the isoform expression pattern of Ca(2+)-regulatory membrane proteins in fast-twitch, slow-twitch, cardiac, neonatal and chronic low-frequency stimulated muscle fibers.
Froemming GR, Murray BE, Harmon S, Pette D, Ohlendieck K
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Biochimica et biophysica acta 2000 Jun 1;1466(1-2):151-68
Two isoforms of sarco/endoplasmic reticulum calcium ATPase (SERCA) are essential in Caenorhabditis elegans.
Cho JH, Bandyopadhyay J, Lee J, Park CS, Ahnn J
Gene 2000 Dec 31;261(2):211-9
Gene 2000 Dec 31;261(2):211-9
Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle.
Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schöneich C
The Biochemical journal 1999 Jun 15;340 ( Pt 3)(Pt 3):657-69
The Biochemical journal 1999 Jun 15;340 ( Pt 3)(Pt 3):657-69
Type 3 and type 1 ryanodine receptors are localized in triads of the same mammalian skeletal muscle fibers.
Flucher BE, Conti A, Takeshima H, Sorrentino V
The Journal of cell biology 1999 Aug 9;146(3):621-30
The Journal of cell biology 1999 Aug 9;146(3):621-30
Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function.
Buck ED, Nguyen HT, Pessah IN, Allen PD
The Journal of biological chemistry 1997 Mar 14;272(11):7360-7
The Journal of biological chemistry 1997 Mar 14;272(11):7360-7
Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function.
Buck ED, Nguyen HT, Pessah IN, Allen PD
The Journal of biological chemistry 1997 Mar 14;272(11):7360-7
The Journal of biological chemistry 1997 Mar 14;272(11):7360-7
Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle.
Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ
The Journal of cell biology 1997 Feb 10;136(3):621-31
The Journal of cell biology 1997 Feb 10;136(3):621-31
Transcriptional regulation of phospholamban gene and translational regulation of SERCA2 gene produces coordinate expression of these two sarcoplasmic reticulum proteins during skeletal muscle phenotype switching.
Hu P, Yin C, Zhang KM, Wright LD, Nixon TE, Wechsler AS, Spratt JA, Briggs FN
The Journal of biological chemistry 1995 May 12;270(19):11619-22
The Journal of biological chemistry 1995 May 12;270(19):11619-22
Transcriptional regulation of phospholamban gene and translational regulation of SERCA2 gene produces coordinate expression of these two sarcoplasmic reticulum proteins during skeletal muscle phenotype switching.
Hu P, Yin C, Zhang KM, Wright LD, Nixon TE, Wechsler AS, Spratt JA, Briggs FN
The Journal of biological chemistry 1995 May 12;270(19):11619-22
The Journal of biological chemistry 1995 May 12;270(19):11619-22
Characterization and ultrastructural localization of a novel 90-kDa protein unique to skeletal muscle junctional sarcoplasmic reticulum.
Guo W, Jorgensen AO, Campbell KP
The Journal of biological chemistry 1994 Nov 11;269(45):28359-65
The Journal of biological chemistry 1994 Nov 11;269(45):28359-65
Biochemical characterization of ultrastructural localization of a major junctional sarcoplasmic reticulum glycoprotein (triadin).
Knudson CM, Stang KK, Jorgensen AO, Campbell KP
The Journal of biological chemistry 1993 Jun 15;268(17):12637-45
The Journal of biological chemistry 1993 Jun 15;268(17):12637-45
Immunological relatedness of the sarcoplasmic reticulum Ca(2+)-ATPase and the Na+,K(+)-ATPase.
Molnar E, Varga S, Jona I, Seidler NW, Martonosi A
Biochimica et biophysica acta 1992 Jan 31;1103(2):281-95
Biochimica et biophysica acta 1992 Jan 31;1103(2):281-95
Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma.
Ohlendieck K, Ervasti JM, Snook JB, Campbell KP
The Journal of cell biology 1991 Jan;112(1):135-48
The Journal of cell biology 1991 Jan;112(1):135-48
Analysis of excitation-contraction-coupling components in chronically stimulated canine skeletal muscle.
Ohlendieck K, Briggs FN, Lee KF, Wechsler AW, Campbell KP
European journal of biochemistry 1991 Dec 18;202(3):739-47
European journal of biochemistry 1991 Dec 18;202(3):739-47
Analysis of excitation-contraction-coupling components in chronically stimulated canine skeletal muscle.
Ohlendieck K, Briggs FN, Lee KF, Wechsler AW, Campbell KP
European journal of biochemistry 1991 Dec 18;202(3):739-47
European journal of biochemistry 1991 Dec 18;202(3):739-47
Ca-ATPase isozyme expression in sarcoplasmic reticulum is altered by chronic stimulation of skeletal muscle.
Briggs FN, Lee KF, Feher JJ, Wechsler AS, Ohlendieck K, Campbell K
FEBS letters 1990 Jan 1;259(2):269-72
FEBS letters 1990 Jan 1;259(2):269-72
The binding of monoclonal and polyclonal antibodies to the Ca2(+)-ATPase of sarcoplasmic reticulum: effects on interactions between ATPase molecules.
Molnar E, Seidler NW, Jona I, Martonosi AN
Biochimica et biophysica acta 1990 Apr 13;1023(2):147-67
Biochimica et biophysica acta 1990 Apr 13;1023(2):147-67
A monoclonal antibody to the Ca2+-ATPase of cardiac sarcoplasmic reticulum cross-reacts with slow type I but not with fast type II canine skeletal muscle fibers: an immunocytochemical and immunochemical study.
Jorgensen AO, Arnold W, Pepper DR, Kahl SD, Mandel F, Campbell KP
Cell motility and the cytoskeleton 1988;9(2):164-74
Cell motility and the cytoskeleton 1988;9(2):164-74
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
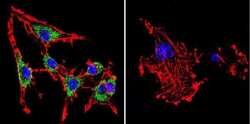
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (IIH11) (Product # MA3-911) shows staining in C6 Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-911) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
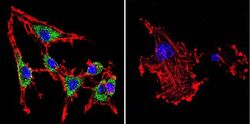
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (IIH11) (Product # MA3-911) shows staining in C6 Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-911) at a dilution of 1:200 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
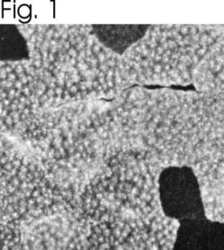
- Experimental details
- Immunolocalization of SERCA1 ATPase in canine skeletal muscle using Product # MA3-911.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
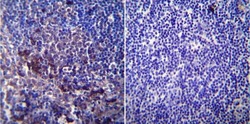
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse lymph node tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing SERCA1 ATPase (Product # MA3-911) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
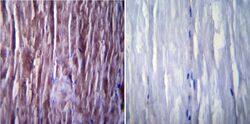
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse skeletal muscle tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing SERCA1 ATPase (Product # MA3-911) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
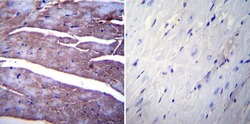
- Experimental details
- Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse heart tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing SERCA1 ATPase (Product # MA3-911) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
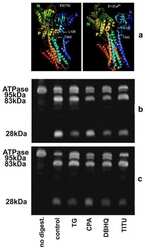
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
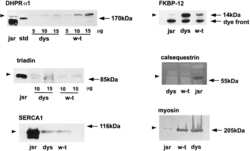
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
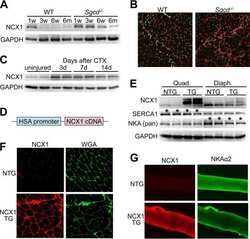
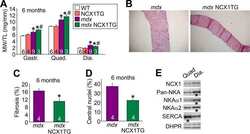
- Figure 3 Ca 2+ handling proteins. (A) Representative western blot images of Ca V 1.1, RyR1, SERCA1, SERCA2, calsequestrin (1 and 2), and sarcolipin. To allow the use of data in multiple western blots each band within a single western blot was normalized to GAPDH for that sample as a loading control and then normalized to the average WT values for that particular gel to give %WT. (B) Analysis of muscle levels of Ca V 1.1 normalized to GAPDH. (C) Analysis of muscle levels of RyR1 normalized to GAPDH. (D) Analysis of muscle levels of SERCA1 normalized to GAPDH. (E) Analysis of muscle levels of SERCA2 normalized to GAPDH. (F) Analysis of muscle levels of CSQ normalized to GAPDH. (G) Analysis of sarcolipin normalized to GAPDH. (H) SERCA activity as a function of Ca 2+ concentration. (I) Scheme of changes in Ca 2+ handling proteins. Values are shown as mean +- SEM. * P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2 Protein levels of sarcoplasmic reticulum Ca 2+ -handling proteins in intrinsic laryngeal muscles (ILM), cricothyroid (CT) muscle, extraocular muscles (EOM), and tibialis anterior (TA) muscle. Western blot analysis showing relative abundance of indicated proteins: calsequestrins 1 and 2 (CASQ1 and CASQ2), Sercas 1 and 2 (SERCA1 and SERCA2), calmodulin (CaM), calmodulin kinase II (CaMKII), and Orai1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for protein loading, Western blot transfer and nonspecific changes in protein levels. The molecular weight, expressed in kDa, for each protein is indicated. Quantifications from three independent muscle samples, each sample containing muscles pooled from three or four different rats. Asterisks and different letter combination indicate statistical significance (* P < 0.05 and ab, ac, ad, bc, or bd P < 0.05, respectively). In ILM, CASQ 1 was more abundant than CASQ2 compared with TA. SERCA1 was less than SERCA2 in ILM and higher compared with TA. CT and the other ILM showed similar levels of the proteins studied.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
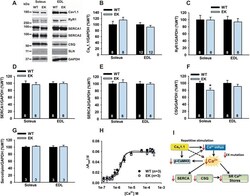
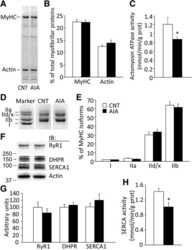
- Fig. 1 Actomyosin ATPase and sarcoplasmic reticulum (SR) Ca 2+ -ATPase activities are decreased in AIA EDL muscles. Representative Coomassie brilliant blue staining of myofibrillar proteins ( a ), percentage distribution of myosin heavy chain (MyHC) and actin content in total myofibrillar proteins ( b ), and actomyosin ATPase activity ( c ). Electrophoretically separated MyHC isoforms ( d ) and percentage distribution of MyHC isoforms ( e ): I, slow isoform and IIa, IId/x, and IIb, fast isoforms. Representative Western blots of ryanodine receptor (RyR1), dihydropyridine receptor alpha2 subunit (DHPR), and SR Ca 2+ -ATPase (SERCA) 1 ( f ), the expression levels of these proteins normalized to actin content ( g ), and SERCA activity ( h ). Control (CNT), white bars ; AIA, black bars . Data are presented as mean +- SEM for three to seven muscles in each group. * P < 0.05 versus CNT
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
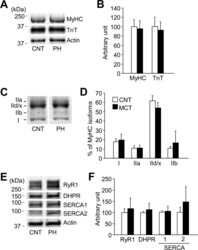
- Fig 2 Neither contractile proteins nor excitation-contraction coupling proteins are altered in PH diaphragm muscles. A : Stain free images of myosin heavy chain (MyHC) and western blots of troponin (Tn) T. B : the expression levels of MyHC or TnT normalized to actin content. C : electrophoretically separated MyHC isoforms. D : percentage distribution of MyHC isoforms: I, slow myosin isoform; IIa, IId/x, and IIb, fast myosin isoforms. E : representative western blots of ryanodine receptor (RyR1), dihydropyridine receptor alpha2 subunit (DHPR), sarcoplasmic reticulum Ca 2+ -ATPase (SERCA) 1, and SERCA2. F : the expression levels of RyR1, DHPR, SERCA1, or SERCA2 normalized to actin content. Control (CNT), white bars; AIA, black bars. Data represent mean +- SD for 4-8 rats in each group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
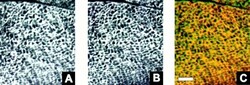
- Figure 9 Comparison of the distribution of the small ankyrins and the SERCA1 ATPase in rat skeletal muscle. Frozen cross sections (5 mum) of the extensor digitorum longus muscle of the rat were prepared and double-labeled by immunofluorescence with anti-p6 antibodies to the small ankyrins and monoclonal antibodies to the SERCA1 ATPase of fast twitch muscle fibers, as described in Materials and Methods. Images were obtained by confocal microscopy. ( A ) SERCA ATPase, visualized with fluoresceinated anti-mouse IgG. ( B ) Small ankyrins, visualized with tetramethylrhodamine-conjugated anti-rabbit IgG. ( C ) Computer overlay of the images in A and B , in which structures labeled by both antibodies appear yellow. There is extensive coincidence of the two labels. Bar, 6 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
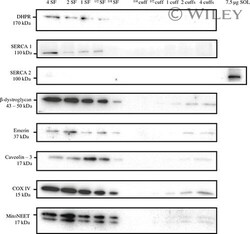
- 3 Representative Western blots performed on mechanically skinned fiber equivalents and sarcolemmal cuffs probing for various subcellular membrane markers. Transverse-tubules, DHPR; sarcoplasmic reticulum, SERCA1 and 2; sarcolemma, beta-dystroglycan; nucleus, emerin; caveolar, caveolin-3; inner mitochondria, COX IV; outer mitochondria, mitoNEET. SF, mechanically skinned fiber equivalent; cuff, sarcolemmal cuff equivalent; SOL, soleus muscle protein
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
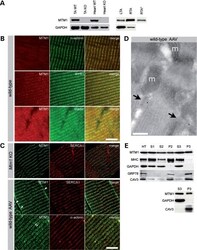
- Figure 5. Subcellular localization of myotubularin in skeletal muscle . (A) Detection of endogenous myotubularin by western blot. Left panel shows the presence of myotubularin in wild-type (WT) but not KO skeletal muscle and heart. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) immunoreactivity was used as an internal control. Right panel illustrates the level of expression of myotubularin 6 weeks after adeno-associated virus (AAV) injection; 75 and 7.5 ug of proteins were loaded for phosphate-buffered saline (PBS) (LTA) and AAV (RTA)-injected WT muscles, respectively. A lower exposure of myotubularin band in RTA (RTA*) is shown to illustrate the doublet. ( B ) Localization of endogenous myotubularin in skeletal muscle. Semithin cryosections (0.5 um) of WT muscle were stained for MTM1 (myotubularin), alpha-actinin (Z-lines), ryanodine receptor (RYR) and triadin (both in triads). Occasional alpha-actinin-positive Z-lines appear yellow when oblique orientation of the sarcomeres in sections superimpose the Z-lines with the adjacent triadic regions. The bar represents 5 um. ( C ) Localization of overexpressed myotubularin in skeletal muscle. Semithin Mtm1 -deficient (upper panels) and WT-AAV (middle and lower panels) muscle cryosections were stained for MTM1, SERCA1 (sarco-endoplasmic reticulum calcium ATPase 1) and alpha-actinin. Myotubularin antibody binds aspecifically to a region between I-bands in WT and KO myofibers, which probably corresponds to the M-band. Note myotubul
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
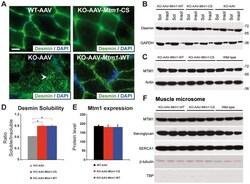
- Figure 7 The phosphatase-dead C375S myotubularin mutant injection in Mtm1 -KO muscle restores normal desmin expression and localization. (A) Ectopic expression of MTM1 transgene in Mtm1 -KO muscle restored normal desmin localization in muscle. Arrowheads indicate aggregates of desmin in Mtm1 -KO muscle injected with AAV. Scale bars: 10 um. (B) The phosphatase-dead C375S myotubularin mutant expression in Mtm1 -KO muscle restored normal desmin expression level in soluble and insoluble fraction. (C) Skeletal muscle protein lysates 4 weeks post-injection were immunoblotted for MTM1. (D) Quantification of relative desmin expression level in soluble compared to insoluble fraction. Data correlated from 2 independent experiments (n = 3 mice per group). *P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
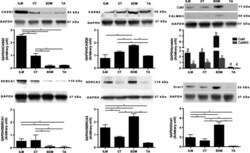
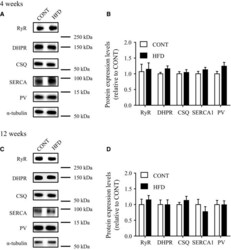
- Figure 5 Expression levels of muscle calcium-regulated proteins in mice after a high-fat diet. Protein levels in control mice ( CONT ) and high-fat diet-fed mice ( HFD ; upper panel: 4 weeks; lower panels: 12 weeks). Left: representative western blots of RyR, DHPR , CSQ , SERCA 1 and PV proteins (A: 4 weeks; C: 12 weeks). A protein expression levels expressed relative to the value of CONT mice (B: 4 weeks; D: 12 weeks). Values are means +- SE ( n = 7).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
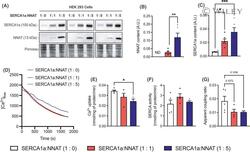
- 2 Fig. NNAT reduces SERCA1a-mediated Ca 2+ uptake. (A) Representative Western blot images of NNAT and SERCA1a in HEK co-transfected cells. Ponceau staining was used as a loading control. (B) NNAT and (C) SERCA2a protein content in 1 : 0, 1 : 1, and 1 : 5 SERCA : NNAT co-transfected cells. (D) Change in [Ca 2+ ] free after the initiation of Ca 2+ uptake without oxalate in the buffer in the 1 : 0, 1 : 1, and 1 : 5 SERCA : NNAT co-transfected cells. (E) Rates of Ca 2+ uptake and (F) SERCA ATP hydrolysis (without ionophore) analyzed at a [Ca 2+ ] free of 1500 n m . (G) The calculated apparent coupling ratio (Ca 2+ uptake/SERCA activity) in 1 : 0, 1 : 1, and 1 : 5 (cDNA) SERCA : NNAT co-transfected cells. Data are means +- SEM; unpaired Student's t -test (B); one-way ANOVA (F) followed by a Tukey's post-hoc (C, E, G). * P < 0.05; ** P < 0.01; *** P < 0.005 ( n = 4-6 per group, technical replicates from passages 8-12). Values above bars indicate P value.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
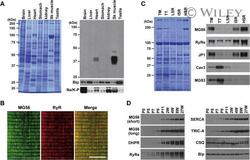
- Biochemical characterization of MG56. (A) Western blot analysis of mouse tissue microsomes using antibody specific to MG56. Total microsomal proteins from mouse tissues (5 mug protein/lane) were separated by SDS-PAGE and Coomassie staining (left panel), and also subjected to immunoblot analysis using antibody specific to MG56 (right panel). Bip and Na-K pump (Na/K-P) were also detected as loading markers. (B) Immunohistochemical detection of MG56 in mouse skeletal muscle. A longitudinal section was stained with antibodies to MG56 and RyR, and subjected to confocal microscopic observation. Scale bar, 10 mum. (C) Distribution of MG56 in muscle membrane preparations. Total microsomal (TM), T-tubular (TT), light SR (LSR), intermediate SR (ISR) and heavy SR (HSR) fractions were prepared from mouse skeletal muscle. The membrane preparations (5 mug/lane) were analyzed by SDS-PAGE and Coomassie staining (left panel), and also subjected to immunoblot analysis using antibodies specific to MG56 and maker proteins (right panel). RyRs (RyR1 and RyR3) and JP1 are heavy SR markers, while caveolin-3 (Cav3) and MG53 are T-tubular markers. (D) Postnatal MG56 expression. Total muscle microsomes were prepared from mice at various developmental stages, from neonates (P0) to 27-week-old mice (27W). Microsomal proteins were analyzed by Western blotting using antibodies specific to MG56, RyRs, DHPR, JP1, triadin, SERCA1, TRIC-A and BiP/GRP78.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
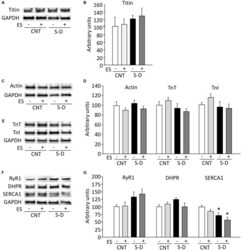
- FIGURE 5 Expressions of thin filament and sarcoplasmic reticulum Ca 2+ handling proteins are unaltered in S-D muscles. Representative immunoblots of myosin filament-linked protein titin (A) , thin filament proteins actin (C) , troponin (Tn) T, TnI (D) , sarcoplasmic reticulum Ca 2+ handling proteins ryanodine receptor (RyR) 1, DHPR-alpha2 subunit, and sarcoplasmic reticulum Ca 2+ -ATPase (SERCA) 1 (F) . The expression levels of titin (B) , actin, TnT, TnI (E) , RyR1, DHPR, and SERCA1 (G) normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) content. Results are expressed as a percentage of CNT value. Bars show the mean and SEM results from 5 to 6 muscles per group. * P < 0.05 vs. CNT.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
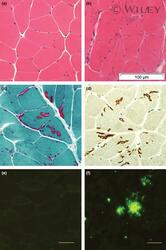
- (a) Normal muscle from a 12 month female transgenic mouse (H&E); (b) Multiple tubular aggregates in muscle fibres from an 18 month old male transgenic mouse (H&E); (c) Tubular aggregates demonstrated on Gomori-Trichrome stain; (D) Tubular aggregates are positive for SERCA 1- ATP ase immunostaining; (e) Muscle fibres from a 12 month old transgenic animal does not show immuno-reactivity for Abeta PP /Abeta; (f) Abeta PP /Abeta-positive aggregates in tg2576 mouse brain tissue. Magnification: (a-d) x400, (e,f) x200. Antibody for (e,f): 6E10.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry