Antibody data
- Antibody Data
- Antigen structure
- References [77]
- Comments [0]
- Validations
- Western blot [2]
- Immunocytochemistry [5]
- Immunohistochemistry [3]
- Other assay [37]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-912 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- SERCA1 ATPase Monoclonal Antibody (VE121G9)
- Antibody type
- Monoclonal
- Antigen
- Purifed from natural sources
- Description
- MA3-912 detects sarcoplasmic or endoplasmic reticulum calcium 1 (SERCA1) ATPase in canine, amphibian, human, mouse, rat, and rabbit tissues. MA3-912 has been successfully used in Western blot and immunofluorescence procedures. By Western blot, this antibody detects an ~110 kDa protein representing SERCA1 ATPase in mouse muscle extracts. Immunofluorescence staining of SERCA1 ATPase in canine skeletal muscle with MA3-912 results in strong labeling of the entire type II (fast) myofiber. MA3-912 has also been shown to inhibit the crystallization of SERCA ATPase induced by vanadate. The MA3-912 antigen is purified rabbit skeletal muscle sarcoplasmic reticulum. This antibody recognizes an epitope between amino acid residues 506 and the C-terminus of rabbit skeletal muscle ATPase, a region that is exposed in native sarcoplasmic reticulum.
- Reactivity
- Human, Mouse, Rat, Canine, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- VE121G9
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Quantification of the calcium signaling deficit in muscles devoid of triadin.
The effects of neurogranin knockdown on SERCA pump efficiency in soleus muscles of female mice fed a high fat diet.
Targeting the Ubiquitin-Proteasome System in Limb-Girdle Muscular Dystrophy With CAPN3 Mutations.
Heterozygous SOD2 deletion selectively impairs SERCA function in the soleus of female mice.
Oxygen Consumption and Basal Metabolic Rate as Markers of Susceptibility to Malignant Hyperthermia and Heat Stroke.
Parvalbumin affects skeletal muscle trophism through modulation of mitochondrial calcium uptake.
β(2)-Adrenergic agonist salbutamol augments hypertrophy in MHCIIa fibers and sprint mean power output but not muscle force during 11 weeks of resistance training in young men.
Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans.
Intracellular calcium leak lowers glucose storage in human muscle, promoting hyperglycemia and diabetes.
Inclusion of sprints in moderate intensity continuous training leads to muscle oxidative adaptations in trained individuals.
Effect of speed endurance training and reduced training volume on running economy and single muscle fiber adaptations in trained runners.
Comparative proteomic profiling reveals a role for Cisd2 in skeletal muscle aging.
Effect of tapering after a period of high-volume sprint interval training on running performance and muscular adaptations in moderately trained runners.
Misregulation of calcium-handling proteins promotes hyperactivation of calcineurin-NFAT signaling in skeletal muscle of DM1 mice.
Effect of increased and maintained frequency of speed endurance training on performance and muscle adaptations in runners.
Muscle variables of importance for physiological performance in competitive football.
The maintenance ability and Ca(2+) availability of skeletal muscle are enhanced by sildenafil.
Calsequestrins in skeletal and cardiac muscle from adult Danio rerio.
Calpain 3 deficiency affects SERCA expression and function in the skeletal muscle.
The Effect of SERCA1b Silencing on the Differentiation and Calcium Homeostasis of C2C12 Skeletal Muscle Cells.
Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men.
Functional analysis of SERCA1b, a highly expressed SERCA1 variant in myotonic dystrophy type 1 muscle.
Histological characterization and biochemical analysis of paraspinal muscles in neuromuscularly healthy subjects.
Unchanged content of oxidative enzymes in fast-twitch muscle fibers and V˙O2 kinetics after intensified training in trained cyclists.
Reelin protects against amyloid β toxicity in vivo.
Concurrent speed endurance and resistance training improves performance, running economy, and muscle NHE1 in moderately trained runners.
Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice.
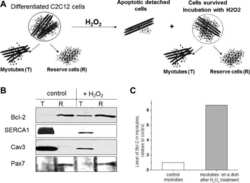
Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes.
Fibre type-specific change in FXYD1 phosphorylation during acute intense exercise in humans.
Training-induced acceleration of O(2) uptake on-kinetics precedes muscle mitochondrial biogenesis in humans.
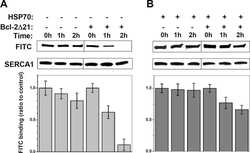
Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: possible implications for the ER Ca2+-mediated apoptosis.
Endurance training decreases the non-linearity in the oxygen uptake-power output relationship in humans.
Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson's disease.
Four weeks of normobaric "live high-train low" do not alter muscular or systemic capacity for maintaining pH and K⁺ homeostasis during intense exercise.
Variation in expression of calcium-handling proteins is associated with inter-individual differences in mechanical performance of rat (Rattus norvegicus) skeletal muscle.
Slowed relaxation and preserved maximal force in soleus muscles of mice with targeted disruption of the Serca2 gene in skeletal muscle.
Identification of secondary effects of hyperexcitability by proteomic profiling of myotonic mouse muscle.
Training effects on skeletal muscle calcium handling in human chronic heart failure.
DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue.
Proteomic profiling of naturally protected extraocular muscles from the dystrophin-deficient mdx mouse.
Immunohistochemical evidence for expression of fast-twitch type sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA1) in German shepherd dogs with dilated cardiomyopathy myocardium.
Altered contractility of skeletal muscle in mice deficient in titin's M-band region.
Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle.
Training induced decrease in oxygen cost of cycling is accompanied by down-regulation of SERCA expression in human vastus lateralis muscle.
Reduced expression of sarcalumenin and related Ca2+ -regulatory proteins in aged rat skeletal muscle.
Conformational fluctuations of the Ca2+-ATPase in the native membrane environment. Effects of pH, temperature, catalytic substrates, and thapsigargin.
Intermolecular interactions in the mechanism of skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase (SERCA1): evidence for a triprotomer.
Effects of high-affinity inhibitors on partial reactions, charge movements, and conformational States of the Ca2+ transport ATPase (sarco-endoplasmic reticulum Ca2+ ATPase).
TRPC3 channels colocalize with Na+/Ca2+ exchanger and Na+ pump in axial component of transverse-axial tubular system of rat ventricle.
Microarray profiling of skeletal muscle sarcoplasmic reticulum proteins.
Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males.
Comparative effects of a low-carbohydrate diet and exercise plus a low-carbohydrate diet on muscle sarcoplasmic reticulum responses in males.
Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue.
Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation.
Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle.
Effect of chronic obstructive pulmonary disease on calcium pump ATPase expression in human diaphragm.
Metabolic and sarcoplasmic reticulum Ca2+ cycling responses in human muscle 4 days following prolonged exercise.
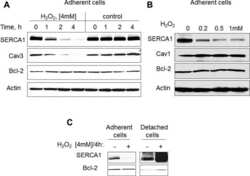
Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA).
The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm.
Increased sensitivity of the ryanodine receptor to halothane-induced oligomerization in malignant hyperthermia-susceptible human skeletal muscle.
Isometric force and endurance in skeletal muscle of mice devoid of all known thyroid hormone receptors.
Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling.
Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling.
Paradoxical effects of prior activity on human sarcoplasmic reticulum Ca2+-ATPase response to exercise.
TGF-beta-induced Ca(2+) influx involves the type III IP(3) receptor and regulates actin cytoskeleton.
Contraction and intracellular Ca(2+) handling in isolated skeletal muscle of rats with congestive heart failure.
Contraction and intracellular Ca(2+) handling in isolated skeletal muscle of rats with congestive heart failure.
Low temperature molecular adaptation of the skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase 1 (SERCA 1) in the wood frog (Rana sylvatica).
Mutations of either or both Cys876 and Cys888 residues of sarcoplasmic reticulum Ca2+-ATPase result in a complete loss of Ca2+ transport activity without a loss of Ca2+-dependent ATPase activity. Role of the CYS876-CYS888 disulfide bond.
Isometric force and endurance in soleus muscle of thyroid hormone receptor-alpha(1)- or -beta-deficient mice.
Isometric force and endurance in soleus muscle of thyroid hormone receptor-alpha(1)- or -beta-deficient mice.
Enhanced sarcoplasmic reticulum Ca(2+) release following intermittent sprint training.
Enhanced sarcoplasmic reticulum Ca(2+) release following intermittent sprint training.
Deletions or specific substitutions of a few residues in the NH(2)-terminal region (Ala(3) to Thr(9)) of sarcoplasmic reticulum Ca(2+)-ATPase cause inactivation and rapid degradation of the enzyme expressed in COS-1 cells.
Immunological relatedness of the sarcoplasmic reticulum Ca(2+)-ATPase and the Na+,K(+)-ATPase.
The binding of monoclonal and polyclonal antibodies to the Ca2(+)-ATPase of sarcoplasmic reticulum: effects on interactions between ATPase molecules.
Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis.
Manno C, Tammineni E, Figueroa L, Marty I, Ríos E
PloS one 2022;17(2):e0264146
PloS one 2022;17(2):e0264146
The effects of neurogranin knockdown on SERCA pump efficiency in soleus muscles of female mice fed a high fat diet.
Braun JL, Ryoo J, Goodwin K, Copeland EN, Geromella MS, Baranowski RW, MacPherson REK, Fajardo VA
Frontiers in endocrinology 2022;13:957182
Frontiers in endocrinology 2022;13:957182
Targeting the Ubiquitin-Proteasome System in Limb-Girdle Muscular Dystrophy With CAPN3 Mutations.
Lasa-Elgarresta J, Mosqueira-Martín L, González-Imaz K, Marco-Moreno P, Gerenu G, Mamchaoui K, Mouly V, López de Munain A, Vallejo-Illarramendi A
Frontiers in cell and developmental biology 2022;10:822563
Frontiers in cell and developmental biology 2022;10:822563
Heterozygous SOD2 deletion selectively impairs SERCA function in the soleus of female mice.
Braun JL, Messner HN, Cleverdon REG, Baranowski RW, Hamstra SI, Geromella MS, Stuart JA, Fajardo VA
Physiological reports 2022 May;10(10):e15285
Physiological reports 2022 May;10(10):e15285
Oxygen Consumption and Basal Metabolic Rate as Markers of Susceptibility to Malignant Hyperthermia and Heat Stroke.
Serano M, Pietrangelo L, Paolini C, Guarnier FA, Protasi F
Cells 2022 Aug 9;11(16)
Cells 2022 Aug 9;11(16)
Parvalbumin affects skeletal muscle trophism through modulation of mitochondrial calcium uptake.
Butera G, Vecellio Reane D, Canato M, Pietrangelo L, Boncompagni S, Protasi F, Rizzuto R, Reggiani C, Raffaello A
Cell reports 2021 May 4;35(5):109087
Cell reports 2021 May 4;35(5):109087
β(2)-Adrenergic agonist salbutamol augments hypertrophy in MHCIIa fibers and sprint mean power output but not muscle force during 11 weeks of resistance training in young men.
Jessen S, Reitelseder S, Kalsen A, Kreiberg M, Onslev J, Gad A, Ørtenblad N, Backer V, Holm L, Bangsbo J, Hostrup M
Journal of applied physiology (Bethesda, Md. : 1985) 2021 Mar 1;130(3):617-626
Journal of applied physiology (Bethesda, Md. : 1985) 2021 Mar 1;130(3):617-626
Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans.
Monti E, Reggiani C, Franchi MV, Toniolo L, Sandri M, Armani A, Zampieri S, Giacomello E, Sarto F, Sirago G, Murgia M, Nogara L, Marcucci L, Ciciliot S, Šimunic B, Pišot R, Narici MV
The Journal of physiology 2021 Jun;599(12):3037-3061
The Journal of physiology 2021 Jun;599(12):3037-3061
Intracellular calcium leak lowers glucose storage in human muscle, promoting hyperglycemia and diabetes.
Tammineni ER, Kraeva N, Figueroa L, Manno C, Ibarra CA, Klip A, Riazi S, Rios E
eLife 2020 May 4;9
eLife 2020 May 4;9
Inclusion of sprints in moderate intensity continuous training leads to muscle oxidative adaptations in trained individuals.
Gunnarsson TP, Brandt N, Fiorenza M, Hostrup M, Pilegaard H, Bangsbo J
Physiological reports 2019 Feb;7(4):e13976
Physiological reports 2019 Feb;7(4):e13976
Effect of speed endurance training and reduced training volume on running economy and single muscle fiber adaptations in trained runners.
Skovgaard C, Christiansen D, Christensen PM, Almquist NW, Thomassen M, Bangsbo J
Physiological reports 2018 Feb;6(3)
Physiological reports 2018 Feb;6(3)
Comparative proteomic profiling reveals a role for Cisd2 in skeletal muscle aging.
Huang YL, Shen ZQ, Wu CY, Teng YC, Liao CC, Kao CH, Chen LK, Lin CH, Tsai TF
Aging cell 2018 Feb;17(1)
Aging cell 2018 Feb;17(1)
Effect of tapering after a period of high-volume sprint interval training on running performance and muscular adaptations in moderately trained runners.
Skovgaard C, Almquist NW, Kvorning T, Christensen PM, Bangsbo J
Journal of applied physiology (Bethesda, Md. : 1985) 2018 Feb 1;124(2):259-267
Journal of applied physiology (Bethesda, Md. : 1985) 2018 Feb 1;124(2):259-267
Misregulation of calcium-handling proteins promotes hyperactivation of calcineurin-NFAT signaling in skeletal muscle of DM1 mice.
Ravel-Chapuis A, Bélanger G, Côté J, Michel RN, Jasmin BJ
Human molecular genetics 2017 Jun 15;26(12):2192-2206
Human molecular genetics 2017 Jun 15;26(12):2192-2206
Effect of increased and maintained frequency of speed endurance training on performance and muscle adaptations in runners.
Skovgaard C, Almquist NW, Bangsbo J
Journal of applied physiology (Bethesda, Md. : 1985) 2017 Jan 1;122(1):48-59
Journal of applied physiology (Bethesda, Md. : 1985) 2017 Jan 1;122(1):48-59
Muscle variables of importance for physiological performance in competitive football.
Mohr M, Thomassen M, Girard O, Racinais S, Nybo L
European journal of applied physiology 2016 Feb;116(2):251-62
European journal of applied physiology 2016 Feb;116(2):251-62
The maintenance ability and Ca(2+) availability of skeletal muscle are enhanced by sildenafil.
Huang M, Lee KJ, Kim KJ, Ahn MK, Cho CH, Kim DH, Lee EH
Experimental & molecular medicine 2016 Dec 9;48(12):e278
Experimental & molecular medicine 2016 Dec 9;48(12):e278
Calsequestrins in skeletal and cardiac muscle from adult Danio rerio.
Furlan S, Mosole S, Murgia M, Nagaraj N, Argenton F, Volpe P, Nori A
Journal of muscle research and cell motility 2016 Apr;37(1-2):27-39
Journal of muscle research and cell motility 2016 Apr;37(1-2):27-39
Calpain 3 deficiency affects SERCA expression and function in the skeletal muscle.
Toral-Ojeda I, Aldanondo G, Lasa-Elgarresta J, Lasa-Fernández H, Fernández-Torrón R, López de Munain A, Vallejo-Illarramendi A
Expert reviews in molecular medicine 2016 Apr 8;18:e7
Expert reviews in molecular medicine 2016 Apr 8;18:e7
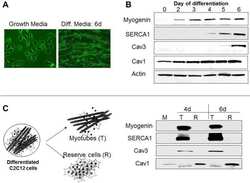
The Effect of SERCA1b Silencing on the Differentiation and Calcium Homeostasis of C2C12 Skeletal Muscle Cells.
Tóth A, Fodor J, Vincze J, Oláh T, Juhász T, Zákány R, Csernoch L, Zádor E
PloS one 2015;10(4):e0123583
PloS one 2015;10(4):e0123583
Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men.
Hostrup M, Kalsen A, Onslev J, Jessen S, Haase C, Habib S, Ørtenblad N, Backer V, Bangsbo J
Journal of applied physiology (Bethesda, Md. : 1985) 2015 Sep 1;119(5):475-86
Journal of applied physiology (Bethesda, Md. : 1985) 2015 Sep 1;119(5):475-86
Functional analysis of SERCA1b, a highly expressed SERCA1 variant in myotonic dystrophy type 1 muscle.
Zhao Y, Ogawa H, Yonekura S, Mitsuhashi H, Mitsuhashi S, Nishino I, Toyoshima C, Ishiura S
Biochimica et biophysica acta 2015 Oct;1852(10 Pt A):2042-7
Biochimica et biophysica acta 2015 Oct;1852(10 Pt A):2042-7
Histological characterization and biochemical analysis of paraspinal muscles in neuromuscularly healthy subjects.
Zimmermann C, Kalepu R, Ponfick M, Reichel H, Cakir B, Zierz S, Gdynia HJ, Kassubek J, Ludolph AC, Rosenbohm A
Muscle & nerve 2015 Jul;52(1):45-54
Muscle & nerve 2015 Jul;52(1):45-54
Unchanged content of oxidative enzymes in fast-twitch muscle fibers and V˙O2 kinetics after intensified training in trained cyclists.
Christensen PM, Gunnarsson TP, Thomassen M, Wilkerson DP, Nielsen JJ, Bangsbo J
Physiological reports 2015 Jul;3(7)
Physiological reports 2015 Jul;3(7)
Reelin protects against amyloid β toxicity in vivo.
Lane-Donovan C, Philips GT, Wasser CR, Durakoglugil MS, Masiulis I, Upadhaya A, Pohlkamp T, Coskun C, Kotti T, Steller L, Hammer RE, Frotscher M, Bock HH, Herz J
Science signaling 2015 Jul 7;8(384):ra67
Science signaling 2015 Jul 7;8(384):ra67
Concurrent speed endurance and resistance training improves performance, running economy, and muscle NHE1 in moderately trained runners.
Skovgaard C, Christensen PM, Larsen S, Andersen TR, Thomassen M, Bangsbo J
Journal of applied physiology (Bethesda, Md. : 1985) 2014 Nov 15;117(10):1097-109
Journal of applied physiology (Bethesda, Md. : 1985) 2014 Nov 15;117(10):1097-109
Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice.
Burr AR, Millay DP, Goonasekera SA, Park KH, Sargent MA, Collins J, Altamirano F, Philipson KD, Allen PD, Ma J, López JR, Molkentin JD
Molecular and cellular biology 2014 Jun;34(11):1991-2002
Molecular and cellular biology 2014 Jun;34(11):1991-2002
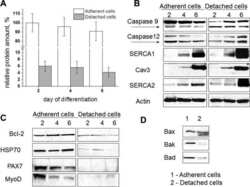
Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes.
Schöneich C, Dremina E, Galeva N, Sharov V
Apoptosis : an international journal on programmed cell death 2014 Jan;19(1):42-57
Apoptosis : an international journal on programmed cell death 2014 Jan;19(1):42-57
Fibre type-specific change in FXYD1 phosphorylation during acute intense exercise in humans.
Thomassen M, Murphy RM, Bangsbo J
The Journal of physiology 2013 Mar 15;591(6):1523-33
The Journal of physiology 2013 Mar 15;591(6):1523-33
Training-induced acceleration of O(2) uptake on-kinetics precedes muscle mitochondrial biogenesis in humans.
Zoladz JA, Grassi B, Majerczak J, Szkutnik Z, Korostyński M, Karasiński J, Kilarski W, Korzeniewski B
Experimental physiology 2013 Apr;98(4):883-98
Experimental physiology 2013 Apr;98(4):883-98
Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: possible implications for the ER Ca2+-mediated apoptosis.
Dremina ES, Sharov VS, Schöneich C
The Biochemical journal 2012 May 15;444(1):127-39
The Biochemical journal 2012 May 15;444(1):127-39
Endurance training decreases the non-linearity in the oxygen uptake-power output relationship in humans.
Majerczak J, Korostynski M, Nieckarz Z, Szkutnik Z, Duda K, Zoladz JA
Experimental physiology 2012 Mar;97(3):386-99
Experimental physiology 2012 Mar;97(3):386-99
Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson's disease.
Wrede A, Margraf NG, Goebel HH, Deuschl G, Schulz-Schaeffer WJ
Acta neuropathologica 2012 Mar;123(3):419-32
Acta neuropathologica 2012 Mar;123(3):419-32
Four weeks of normobaric "live high-train low" do not alter muscular or systemic capacity for maintaining pH and K⁺ homeostasis during intense exercise.
Nordsborg NB, Siebenmann C, Jacobs RA, Rasmussen P, Diaz V, Robach P, Lundby C
Journal of applied physiology (Bethesda, Md. : 1985) 2012 Jun;112(12):2027-36
Journal of applied physiology (Bethesda, Md. : 1985) 2012 Jun;112(12):2027-36
Variation in expression of calcium-handling proteins is associated with inter-individual differences in mechanical performance of rat (Rattus norvegicus) skeletal muscle.
James RS, Walter I, Seebacher F
The Journal of experimental biology 2011 Nov 1;214(Pt 21):3542-8
The Journal of experimental biology 2011 Nov 1;214(Pt 21):3542-8
Slowed relaxation and preserved maximal force in soleus muscles of mice with targeted disruption of the Serca2 gene in skeletal muscle.
Sjåland C, Lunde PK, Swift F, Munkvik M, Ericsson M, Lunde M, Boye S, Christensen G, Ellingsen Ø, Sejersted OM, Andersson KB
The Journal of physiology 2011 Dec 15;589(Pt 24):6139-55
The Journal of physiology 2011 Dec 15;589(Pt 24):6139-55
Identification of secondary effects of hyperexcitability by proteomic profiling of myotonic mouse muscle.
Staunton L, Jockusch H, Wiegand C, Albrecht T, Ohlendieck K
Molecular bioSystems 2011 Aug;7(8):2480-9
Molecular bioSystems 2011 Aug;7(8):2480-9
Training effects on skeletal muscle calcium handling in human chronic heart failure.
Munkvik M, Rehn TA, Slettaløkken G, Hasic A, Hallén J, Sjaastad I, Sejersted OM, Lunde PK
Medicine and science in sports and exercise 2010 May;42(5):847-55
Medicine and science in sports and exercise 2010 May;42(5):847-55
DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue.
Donoghue P, Staunton L, Mullen E, Manning G, Ohlendieck K
Journal of proteomics 2010 Jun 16;73(8):1441-53
Journal of proteomics 2010 Jun 16;73(8):1441-53
Proteomic profiling of naturally protected extraocular muscles from the dystrophin-deficient mdx mouse.
Lewis C, Ohlendieck K
Biochemical and biophysical research communications 2010 Jun 11;396(4):1024-9
Biochemical and biophysical research communications 2010 Jun 11;396(4):1024-9
Immunohistochemical evidence for expression of fast-twitch type sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA1) in German shepherd dogs with dilated cardiomyopathy myocardium.
Summerfield N, Peters ME, Hercock CA, Mobasheri A, Young IS
Journal of veterinary cardiology : the official journal of the European Society of Veterinary Cardiology 2010 Apr;12(1):17-23
Journal of veterinary cardiology : the official journal of the European Society of Veterinary Cardiology 2010 Apr;12(1):17-23
Altered contractility of skeletal muscle in mice deficient in titin's M-band region.
Ottenheijm CA, Hidalgo C, Rost K, Gotthardt M, Granzier H
Journal of molecular biology 2009 Oct 16;393(1):10-26
Journal of molecular biology 2009 Oct 16;393(1):10-26
Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle.
O'Connell K, Ohlendieck K
Proteomics 2009 Dec;9(24):5509-24
Proteomics 2009 Dec;9(24):5509-24
Training induced decrease in oxygen cost of cycling is accompanied by down-regulation of SERCA expression in human vastus lateralis muscle.
Majerczak J, Karasinski J, Zoladz JA
Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2008 Sep;59(3):589-602
Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2008 Sep;59(3):589-602
Reduced expression of sarcalumenin and related Ca2+ -regulatory proteins in aged rat skeletal muscle.
O'Connell K, Gannon J, Doran P, Ohlendieck K
Experimental gerontology 2008 Oct;43(10):958-61
Experimental gerontology 2008 Oct;43(10):958-61
Conformational fluctuations of the Ca2+-ATPase in the native membrane environment. Effects of pH, temperature, catalytic substrates, and thapsigargin.
Inesi G, Lewis D, Toyoshima C, Hirata A, de Meis L
The Journal of biological chemistry 2008 Jan 11;283(2):1189-96
The Journal of biological chemistry 2008 Jan 11;283(2):1189-96
Intermolecular interactions in the mechanism of skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase (SERCA1): evidence for a triprotomer.
Mahaney JE, Thomas DD, Farrance IK, Froehlich JP
Biochemistry 2008 Dec 23;47(51):13711-25
Biochemistry 2008 Dec 23;47(51):13711-25
Effects of high-affinity inhibitors on partial reactions, charge movements, and conformational States of the Ca2+ transport ATPase (sarco-endoplasmic reticulum Ca2+ ATPase).
Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Tal DM, Lewis D, Inesi G
Molecular pharmacology 2008 Apr;73(4):1134-40
Molecular pharmacology 2008 Apr;73(4):1134-40
TRPC3 channels colocalize with Na+/Ca2+ exchanger and Na+ pump in axial component of transverse-axial tubular system of rat ventricle.
Goel M, Zuo CD, Sinkins WG, Schilling WP
American journal of physiology. Heart and circulatory physiology 2007 Feb;292(2):H874-83
American journal of physiology. Heart and circulatory physiology 2007 Feb;292(2):H874-83
Microarray profiling of skeletal muscle sarcoplasmic reticulum proteins.
Schulz JS, Palmer N, Steckelberg J, Jones SJ, Zeece MG
Biochimica et biophysica acta 2006 Sep;1764(9):1429-35
Biochimica et biophysica acta 2006 Sep;1764(9):1429-35
Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males.
Duhamel TA, Perco JG, Green HJ
American journal of physiology. Regulatory, integrative and comparative physiology 2006 Oct;291(4):R1100-10
American journal of physiology. Regulatory, integrative and comparative physiology 2006 Oct;291(4):R1100-10
Comparative effects of a low-carbohydrate diet and exercise plus a low-carbohydrate diet on muscle sarcoplasmic reticulum responses in males.
Duhamel TA, Green HJ, Perco JG, Ouyang J
American journal of physiology. Cell physiology 2006 Oct;291(4):C607-17
American journal of physiology. Cell physiology 2006 Oct;291(4):C607-17
Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue.
Lunde PK, Sejersted OM, Thorud HM, Tønnessen T, Henriksen UL, Christensen G, Westerblad H, Bruton J
Circulation research 2006 Jun 23;98(12):1514-9
Circulation research 2006 Jun 23;98(12):1514-9
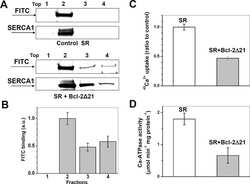
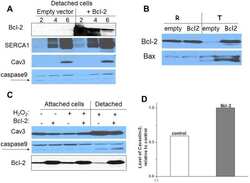
Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation.
Dremina ES, Sharov VS, Schöneich C
Biochemistry 2006 Jan 10;45(1):175-84
Biochemistry 2006 Jan 10;45(1):175-84
Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle.
Rudolf R, Magalhães PJ, Pozzan T
The Journal of cell biology 2006 Apr 24;173(2):187-93
The Journal of cell biology 2006 Apr 24;173(2):187-93
Effect of chronic obstructive pulmonary disease on calcium pump ATPase expression in human diaphragm.
Nguyen T, Rubinstein NA, Vijayasarathy C, Rome LC, Kaiser LR, Shrager JB, Levine S
Journal of applied physiology (Bethesda, Md. : 1985) 2005 Jun;98(6):2004-10
Journal of applied physiology (Bethesda, Md. : 1985) 2005 Jun;98(6):2004-10
Metabolic and sarcoplasmic reticulum Ca2+ cycling responses in human muscle 4 days following prolonged exercise.
Duhamel TA, Green HJ, Perco JG, Ouyang J
Canadian journal of physiology and pharmacology 2005 Jul;83(7):643-55
Canadian journal of physiology and pharmacology 2005 Jul;83(7):643-55
Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA).
Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schöneich C
The Biochemical journal 2004 Oct 15;383(Pt 2):361-70
The Biochemical journal 2004 Oct 15;383(Pt 2):361-70
The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm.
Radzyukevich TL, Moseley AE, Shelly DA, Redden GA, Behbehani MM, Lingrel JB, Paul RJ, Heiny JA
American journal of physiology. Cell physiology 2004 Nov;287(5):C1300-10
American journal of physiology. Cell physiology 2004 Nov;287(5):C1300-10
Increased sensitivity of the ryanodine receptor to halothane-induced oligomerization in malignant hyperthermia-susceptible human skeletal muscle.
Glover L, Heffron JJ, Ohlendieck K
Journal of applied physiology (Bethesda, Md. : 1985) 2004 Jan;96(1):11-8
Journal of applied physiology (Bethesda, Md. : 1985) 2004 Jan;96(1):11-8
Isometric force and endurance in skeletal muscle of mice devoid of all known thyroid hormone receptors.
Johansson C, Lunde PK, Gothe S, Lannergren J, Westerblad H
The Journal of physiology 2003 Mar 15;547(Pt 3):789-96
The Journal of physiology 2003 Mar 15;547(Pt 3):789-96
Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling.
Minamisawa S, Wang Y, Chen J, Ishikawa Y, Chien KR, Matsuoka R
The Journal of biological chemistry 2003 Mar 14;278(11):9570-5
The Journal of biological chemistry 2003 Mar 14;278(11):9570-5
Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling.
Minamisawa S, Wang Y, Chen J, Ishikawa Y, Chien KR, Matsuoka R
The Journal of biological chemistry 2003 Mar 14;278(11):9570-5
The Journal of biological chemistry 2003 Mar 14;278(11):9570-5
Paradoxical effects of prior activity on human sarcoplasmic reticulum Ca2+-ATPase response to exercise.
Tupling AR, Green HJ, Roy BD, Grant S, Ouyang J
Journal of applied physiology (Bethesda, Md. : 1985) 2003 Jul;95(1):138-44
Journal of applied physiology (Bethesda, Md. : 1985) 2003 Jul;95(1):138-44
TGF-beta-induced Ca(2+) influx involves the type III IP(3) receptor and regulates actin cytoskeleton.
McGowan TA, Madesh M, Zhu Y, Wang L, Russo M, Deelman L, Henning R, Joseph S, Hajnoczky G, Sharma K
American journal of physiology. Renal physiology 2002 May;282(5):F910-20
American journal of physiology. Renal physiology 2002 May;282(5):F910-20
Contraction and intracellular Ca(2+) handling in isolated skeletal muscle of rats with congestive heart failure.
Lunde PK, Dahlstedt AJ, Bruton JD, Lännergren J, Thorén P, Sejersted OM, Westerblad H
Circulation research 2001 Jun 22;88(12):1299-305
Circulation research 2001 Jun 22;88(12):1299-305
Contraction and intracellular Ca(2+) handling in isolated skeletal muscle of rats with congestive heart failure.
Lunde PK, Dahlstedt AJ, Bruton JD, Lännergren J, Thorén P, Sejersted OM, Westerblad H
Circulation research 2001 Jun 22;88(12):1299-305
Circulation research 2001 Jun 22;88(12):1299-305
Low temperature molecular adaptation of the skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase 1 (SERCA 1) in the wood frog (Rana sylvatica).
Dode L, Van Baelen K, Wuytack F, Dean WL
The Journal of biological chemistry 2001 Feb 9;276(6):3911-9
The Journal of biological chemistry 2001 Feb 9;276(6):3911-9
Mutations of either or both Cys876 and Cys888 residues of sarcoplasmic reticulum Ca2+-ATPase result in a complete loss of Ca2+ transport activity without a loss of Ca2+-dependent ATPase activity. Role of the CYS876-CYS888 disulfide bond.
Daiho T, Yamasaki K, Saino T, Kamidochi M, Satoh K, Iizuka H, Suzuki H
The Journal of biological chemistry 2001 Aug 31;276(35):32771-8
The Journal of biological chemistry 2001 Aug 31;276(35):32771-8
Isometric force and endurance in soleus muscle of thyroid hormone receptor-alpha(1)- or -beta-deficient mice.
Johansson C, Lännergren J, Lunde PK, Vennström B, Thorén P, Westerblad H
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Mar;278(3):R598-603
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Mar;278(3):R598-603
Isometric force and endurance in soleus muscle of thyroid hormone receptor-alpha(1)- or -beta-deficient mice.
Johansson C, Lännergren J, Lunde PK, Vennström B, Thorén P, Westerblad H
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Mar;278(3):R598-603
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Mar;278(3):R598-603
Enhanced sarcoplasmic reticulum Ca(2+) release following intermittent sprint training.
Ortenblad N, Lunde PK, Levin K, Andersen JL, Pedersen PK
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Jul;279(1):R152-60
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Jul;279(1):R152-60
Enhanced sarcoplasmic reticulum Ca(2+) release following intermittent sprint training.
Ortenblad N, Lunde PK, Levin K, Andersen JL, Pedersen PK
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Jul;279(1):R152-60
American journal of physiology. Regulatory, integrative and comparative physiology 2000 Jul;279(1):R152-60
Deletions or specific substitutions of a few residues in the NH(2)-terminal region (Ala(3) to Thr(9)) of sarcoplasmic reticulum Ca(2+)-ATPase cause inactivation and rapid degradation of the enzyme expressed in COS-1 cells.
Daiho T, Yamasaki K, Suzuki H, Saino T, Kanazawa T
The Journal of biological chemistry 1999 Aug 20;274(34):23910-5
The Journal of biological chemistry 1999 Aug 20;274(34):23910-5
Immunological relatedness of the sarcoplasmic reticulum Ca(2+)-ATPase and the Na+,K(+)-ATPase.
Molnar E, Varga S, Jona I, Seidler NW, Martonosi A
Biochimica et biophysica acta 1992 Jan 31;1103(2):281-95
Biochimica et biophysica acta 1992 Jan 31;1103(2):281-95
The binding of monoclonal and polyclonal antibodies to the Ca2(+)-ATPase of sarcoplasmic reticulum: effects on interactions between ATPase molecules.
Molnar E, Seidler NW, Jona I, Martonosi AN
Biochimica et biophysica acta 1990 Apr 13;1023(2):147-67
Biochimica et biophysica acta 1990 Apr 13;1023(2):147-67
Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis.
Knudson CM, Chaudhari N, Sharp AH, Powell JA, Beam KG, Campbell KP
The Journal of biological chemistry 1989 Jan 25;264(3):1345-8
The Journal of biological chemistry 1989 Jan 25;264(3):1345-8
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
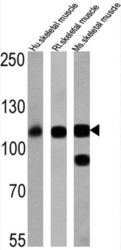
- Experimental details
- Western blot analysis of SERCA1 ATPase was performed by loading 25 µg of human skeletal muscle (lane 1), rat skeletal muscle (lane 2) and mouse skeletal muscle (lane 3) onto an SDS polyacrylamide gel. Proteins were transferred to a PVDF membrane and blocked at 4ºC overnight. The membrane was probed with a SERCA1 ATPase monoclonal antibody (Product # MA3-912) at a dilution of 1:2000 overnight at 4°C, washed in TBST, and probed with an HRP-conjugated secondary antibody for 1 hr at room temperature in the dark. Chemiluminescent detection was performed using Pierce ECL Plus Western Blotting Substrate (Product # 32132). Results show a band at ~110 kDa.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
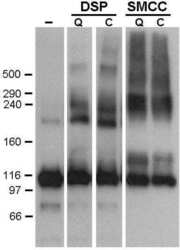
- Western blot was performed using Anti-SERCA1 ATPase Monoclonal Antibody (VE121G9) (Product # MA3-912) and a 95 kDa band corresponding to SERCA1 ATPase was observed across Mouse and Rat skeletal muscle. Tissue extracts (30 µg lysate) of Mouse Skeletal Muscle (Lane 1), Rat Skeletal Muscle (Lane 2), Mouse Heart (Lane 3) and Rat Heart (Lane 4) were electrophoresed using NuPAGE™ 4-12% Bis-Tris Protein Gel (Product # NP0321BOX). Resolved proteins were then transferred onto a Nitrocellulose membrane (Product # LC2002) by iBlot® 2 Dry Blotting System (Product # IB21001). The blot was probed with the primary antibody (1:2500) and detected by chemiluminescence with Goat anti-Mouse IgG (H+L) Superclonal™ Recombinant Secondary Antibody, HRP (Product # A28177, 1:4000) using the iBright FL 1000 (Product # A32752). Chemiluminescent detection was performed using Novex® ECL Chemiluminescent Substrate Reagent Kit (Product # WP20005).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
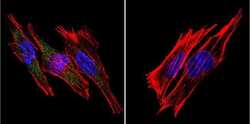
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (VE121G9) (Product # MA3-912) shows staining in A2058 Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-912) at a dilution of 1:20 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
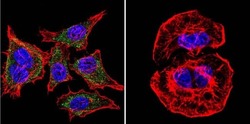
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (VE121G9) (Product # MA3-912) shows staining in Hela Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-912) at a dilution of 1:20 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
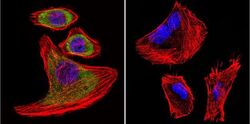
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (VE121G9) (Product # MA3-912) shows staining in U251 Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-912) at a dilution of 1:20 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
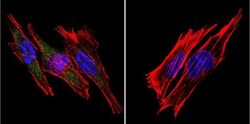
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (VE121G9) (Product # MA3-912) shows staining in A2058 Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-912) at a dilution of 1:20 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
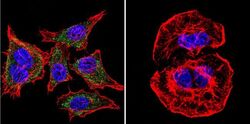
- Experimental details
- Immunofluorescent analysis of SERCA1 ATPase using Anti-SERCA1 ATPase Monoclonal Antibody (VE121G9) (Product # MA3-912) shows staining in Hela Cells. SERCA1 ATPase staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing SERCA1 ATPase (Product # MA3-912) at a dilution of 1:20 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
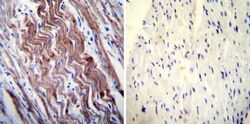
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human heart tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing SERCA1 ATPase (Product # MA3-912) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
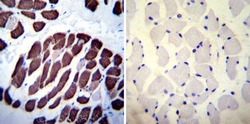
- Experimental details
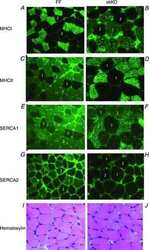
- Immunohistochemistry was performed on normal deparaffinized Human skeletal muscle tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a mouse monoclonal antibody recognizing SERCA1 ATPase (Product # MA3-912) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
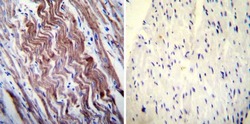
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human heart tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing SERCA1 ATPase (Product # MA3-912) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
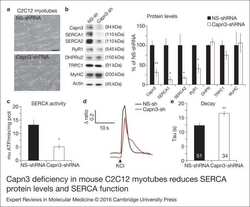
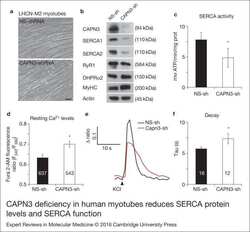
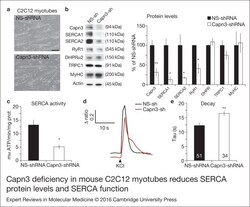
- Figure 1. Capn3 deficiency in mouse C2C12 myotubes reduces SERCA protein levels and SERCAfunction. (a) C2C12 myotubes treated with NS or Capn3 shRNAs and differentiated for7 days. Scale bar = 100 mum (b) Western blot analysis showing significant decreaseof Capn3 (SPA antibody), SERCA1, SERCA2 and RyR1 levels in Capn3 knockdown myotubes,compared with controls (* P < 0.05;** P < 0.01). Total levels of DHPR, TRPC1, MyHC and actin arenot significantly changed. N = 3 independent experiments run on thesame gel. (c) SERCA-specific ATPase activity determined in homogenates from C2C12myotubes. Capn3-deficient myotubes show a significant reduction of SERCA activitycompared with NS-controls ( n = 3,* P < 0.05). (d and e) Ca 2+ imaging of C2C12myotubes loaded with Fura2-AM shows delayed Ca 2+ clearance from thecytosol in Capn3 knockdown myotubes. (d) Two representative traces of changes inFura2-AM fluorescence ratios( F 340 / F 380 ) fromCapn3-shRNA and NS-shRNA treated myotubes. Ca 2+ transients were elicitedby local stimulation with KCl 50 m m in the absence of extracellularCa 2+ . (e) Tau, the time constant of the Ca 2+ transient decayphase in seconds (s), is significantly increased in Capn3-deficient myotubes.** P < 0.01, n = total number of myotubesrecorded from six different experiments are shown in the graph.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
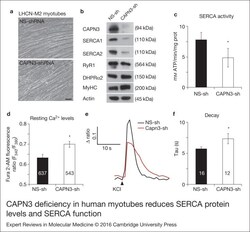
- Figure 2. CAPN3 deficiency in human myotubes reduces SERCA protein levels and SERCA function.(a) LHCN-M2 myoblasts treated with control NS-shRNA or CAPN3-shRNAs anddifferentiated for 9 days. Scale bar = 50 mum. (b) Representative Western blotanalysis showing decrease of CAPN3 (12A2 mAb), SERCA1, SERCA2 and RyR1 proteinlevels in CAPN3-sh treated myotubes compared with controls. DHPRalpha2, MyHC and actinlevels remain unaltered. (c) SERCA-specific ATPase activity determined inhomogenates from LHCN-M2 myotubes. CAPN3-deficient myotubes show a significantreduction of SERCA activity compared with NS-controls ( n = 3,* P < 0.05). D-F) Ca 2+ imaging of LHCN-M2myotubes loaded with Fura2-AM shows increased cytosolic [Ca 2+ ] anddelayed Ca 2+ clearance from the cytosol in CAPN3 knockdown myotubes. (d)Resting cytosolic [Ca 2+ ] was measured in the presence of 2 m m Ca 2+ at 37degC. CAPN3-deficient human myotubes show significantlyincreased resting cytosolic [Ca 2+ ] compared with controls(* P < 0.05; n = 10 experiments). Totalnumbers of myotubes recorded are shown in the graph. (e) Two representative tracesof changes in Fura2-AM fluorescence ratios( F 340 / F 380 ) fromCAPN3-shRNA and NS-shRNA treated myotubes. Ca 2+ transients were elicitedby local stimulation with KCl 130 m m in the absence of extracellularCa 2+ . (f) Tau, the time constant of the Ca 2+ transient decayphase in seconds (s), is significantly increased in CAPN3-deficient myotubes.* P < 0.05, n = total number of myotubesr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
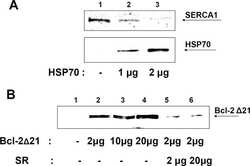
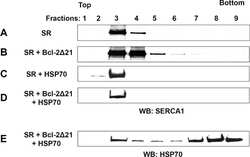
- Fig 1 Effect of SERCA1b shRNA on the expression pattern of proteins involved in Ca 2+ -homeostasis. Examination of decreased SERCA1b expression in C2C12 myotubes. ( A-B ) Protein expression of SERCA was detected by Western-blot analysis to prove the efficiency of SERCA1b-specific shRNA in multinucleated myotubes. Different stably transfected clones, pool of the clones and scrambled shRNA transfected cells were compared. The 115 kDa isoforms were detected either by SERCA1b specific antibody corresponding to the terminal octamer of the protein or by an antibody recognizing both SERCA1a and b isoforms. Actin was used as a control. ( C ) Western blot analysis to detect the SR calcium-binding protein (calsequestrin) expression in selected cloneC1, C5, scrambled shRNA transfected, and parental cells. ( D ) Western-blot analysis showing the protein level of the key molecule of SOCE (STIM1). Total protein samples were used (30 mug in each lane) to examine the protein expression level. For preparing protein samples cultures were harvested on the 5 th day of differentiation in each cases. Representative data of 3 independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 1. Capn3 deficiency in mouse C2C12 myotubes reduces SERCA protein levels and SERCAfunction. (a) C2C12 myotubes treated with NS or Capn3 shRNAs and differentiated for7 days. Scale bar = 100 mum (b) Western blot analysis showing significant decreaseof Capn3 (SPA antibody), SERCA1, SERCA2 and RyR1 levels in Capn3 knockdown myotubes,compared with controls (* P < 0.05;** P < 0.01). Total levels of DHPR, TRPC1, MyHC and actin arenot significantly changed. N = 3 independent experiments run on thesame gel. (c) SERCA-specific ATPase activity determined in homogenates from C2C12myotubes. Capn3-deficient myotubes show a significant reduction of SERCA activitycompared with NS-controls ( n = 3,* P < 0.05). (d and e) Ca 2+ imaging of C2C12myotubes loaded with Fura2-AM shows delayed Ca 2+ clearance from thecytosol in Capn3 knockdown myotubes. (d) Two representative traces of changes inFura2-AM fluorescence ratios( F 340 / F 380 ) fromCapn3-shRNA and NS-shRNA treated myotubes. Ca 2+ transients were elicitedby local stimulation with KCl 50 m m in the absence of extracellularCa 2+ . (e) Tau, the time constant of the Ca 2+ transient decayphase in seconds (s), is significantly increased in Capn3-deficient myotubes.** P < 0.01, n = total number of myotubesrecorded from six different experiments are shown in the graph.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2. CAPN3 deficiency in human myotubes reduces SERCA protein levels and SERCA function.(a) LHCN-M2 myoblasts treated with control NS-shRNA or CAPN3-shRNAs anddifferentiated for 9 days. Scale bar = 50 mum. (b) Representative Western blotanalysis showing decrease of CAPN3 (12A2 mAb), SERCA1, SERCA2 and RyR1 proteinlevels in CAPN3-sh treated myotubes compared with controls. DHPRalpha2, MyHC and actinlevels remain unaltered. (c) SERCA-specific ATPase activity determined inhomogenates from LHCN-M2 myotubes. CAPN3-deficient myotubes show a significantreduction of SERCA activity compared with NS-controls ( n = 3,* P < 0.05). D-F) Ca 2+ imaging of LHCN-M2myotubes loaded with Fura2-AM shows increased cytosolic [Ca 2+ ] anddelayed Ca 2+ clearance from the cytosol in CAPN3 knockdown myotubes. (d)Resting cytosolic [Ca 2+ ] was measured in the presence of 2 m m Ca 2+ at 37degC. CAPN3-deficient human myotubes show significantlyincreased resting cytosolic [Ca 2+ ] compared with controls(* P < 0.05; n = 10 experiments). Totalnumbers of myotubes recorded are shown in the graph. (e) Two representative tracesof changes in Fura2-AM fluorescence ratios( F 340 / F 380 ) fromCAPN3-shRNA and NS-shRNA treated myotubes. Ca 2+ transients were elicitedby local stimulation with KCl 130 m m in the absence of extracellularCa 2+ . (f) Tau, the time constant of the Ca 2+ transient decayphase in seconds (s), is significantly increased in CAPN3-deficient myotubes.* P < 0.05, n = total number of myotubesr
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
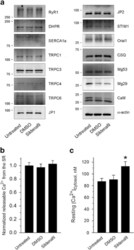
- Figure 5 Expression level of skeletal muscle proteins, the amount of releasable Ca 2+ from the SR to the cytosol, and the resting cytosolic Ca 2+ level in primary skeletal myotubes. ( a ) The immunoblot analysis of proteins mediating Ca 2+ movements and handling in skeletal muscle was conducted using the lysate of myotubes treated with sildenafil. Fifteen proteins were examined, and no expression levels were changed by sildenafil (the expression level of proteins is presented as bar graphs in Supplementary Figure 5 ). alpha-Actin was used as a loading control. At least three independent experiments per protein were conducted and a representative result is presented. JP, junctophilin; CSQ, calsequestrin; Mg, mitsugumin; CaM, calmodulin. The amount of releasable Ca 2+ from the SR to the cytosol in response to TG (2.5 mu M ) ( b ) or resting cytosolic Ca 2+ level ( c ) was examined in myotubes treated with sildenafil, and histograms are shown for the normalized peak amplitude or area under the peak to the mean value of those from untreated controls as described in the 'Materials and methods' section. TG was applied to myotubes in the absence of extracellular Ca 2+ to avoid extracellular Ca 2+ entry. The results are presented as the means+-s.e. for the number of experiments presented in the parentheses of Table 2 . *, and the significant difference was compared with the untreated control ( P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
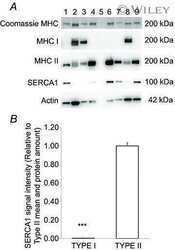
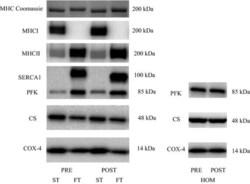
- Figure 3 Representative western blots of the proteins investigated in slow-twitch (ST), fast-twitch (FT), and muscle homogenate (HOM) before (PRE) and after (POST) 7 weeks of high intensity and reduced volume training in trained cyclists. See methods section for details.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
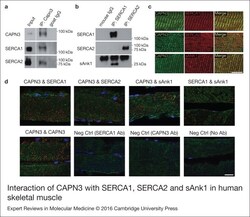
- Figure 5. Interaction of CAPN3 with SERCA1, SERCA2 and sAnk1 in human skeletal muscle. (a)Immunoprecipitation (IP) of CAPN3 with a goat polyclonal antibody (pIS2C) in a vastus lateralis muscle from a healthy donor. Both, SERCA1 andSERCA2 are detected in the CAPN3 IP. White lines indicate noncontiguous lanes run onthe same gel. Input: protein extract. (b) SERCA1 and SERCA2 were immunoprecipitated(IP) with specific monoclonal antibodies in the same muscle. sAnk1 is detected inSERCA1 IP but not in SERCA2 IP. (c) Longitudinal sections from a dorsal human muscleco-immunostained for CAPN3-SERCA1, CAPN3-SERCA2 and CAPN3-sAnk1, showing similardistribution pattern of CAPN3 (green), SERCA1 (red), SERCA2 (red) and sAnk1 (red) inthe panels labelled ""Merge"". Scale bar: 10 um. (d) Co-localisation analysis ofCAPN3, with SERCA1, SERCA2 and sAnk1 in longitudinal sections from human dorsalmuscle using in situ PLA. Red spots represent protein complexes in close proximity(
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
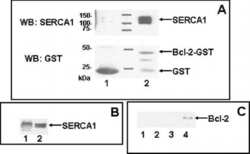
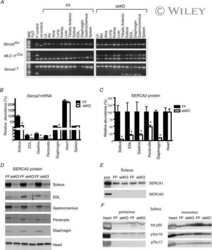
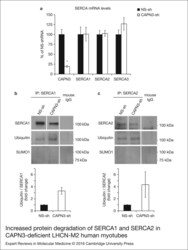
- Figure 6. Increased protein degradation of SERCA1 and SERCA2 in CAPN3-deficient LHCN-M2 humanmyotubes. (a) CAPN3 and SERCA mRNA levels were analysed in human myotubes byquantitative RT-PCR. Statistical analysis showed significant decrease in CAPN3expression (19.3 +- 2.6%) in CAPN3 knockdown myotubes as compared to matched controls(100 +- 12.8%). N = 3; * P < 0.005. Nosignificant changes were observed in SERCA1, SERCA2 or SERCA3 mRNA expressionlevels. (b) SERCA1 and (c) SERCA2 ubiquitination and sumoylation were analysed incontrol and CAPN3-deficient human myotubes through SERCA1/2 immunoprecipitation withspecific mouse monoclonal antibodies. Pools of control and CAPN3-deficient cultureswere used for immunoprecipitation assays. N = 2 and N = 3 independent experiments were performed for SERCA1 and SERCA2,respectively. White lines indicate noncontiguous lanes run on the same gel.Ubiquitination of SERCA1 and SERCA2 in CAPN3-deficient myotubes was increased 3.30and 4.35-fold, respectively, compared with controls. No SUMO1 specific sumoylationof SERCA1 and SERCA2 proteins was detected in controls or CAPN3-deficientmyotubes.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
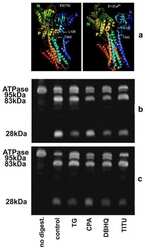
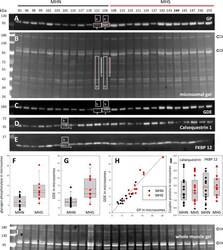
- Figure 2. Protein content in patients' microsomes. ( A ) Western blot analysis of glycogen phosphorylase (GP) for 25 subjects, in a luminescence scale with black as 0 and white as saturating value. The numbers above each lane are patient identifiers that apply to all 25-lane gels shown in the article. The twelve lanes under the black bar have protein from MHN patients; those under the red bar are from MHS patients, except #144, which was reclassified as MHN. ( B ) Ponceau-stained gel that originated all blots in the figure. A and B illustrate our custom quantitative analysis. The content of every protein quantified in blots was calculated as the signal mass within region a above background (average level in b). Content was normalized for quantity of preparation in the lane dividing by the signal similarly calculated in the gel in B. The signal in B is computed in a large area of the lane to average multiple proteins in the fraction. The method is fully demonstrated in Video 1 . Arrows in B mark two bands, near 100 and 180 kDa, with a visibly greater signal in the MHS group. Their main components were GP and glycogen debranching enzyme (GDE) respectively. ( C-E ) Western blots of GDE, calsequestrin 1 and FKBP12 in different sections of gel B (identified by molecular weight markers at left). ( F and G ) Box plots of GP and GDE content. In both cases the content was greater on average for MHS and the differences significant (data in Table 1 ). ( H ) GP vs. GDE in blots A and C;
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
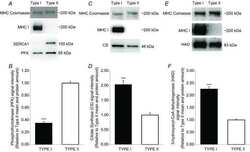
- Figure 5 Decreased activity of Serca1 and increased oxidative stress in naturally and prematurely aged gastrocnemius muscle. (a) Significant reductions occurred in calcium-dependent Serca ATPase activity in naturally aged (26M) WT and prematurely aged (3M) Cisd2 mKO mice. The selective Serca pump inhibitor, TBQ, was used to reflect a specific difference in Serca activity. n = 4 for each group of mice. (b) Increased cysteine S-sulfonation and tyrosine nitration on Serca1 protein in gastrocnemius muscles. The total Serca1 protein was detected by re-blotting on the same membrane. (c,d) Increase in oxidative modifications, namely S-sulfonated cysteine and 3-nitrotyrosine, for all proteins present in whole cell extracts of the gastrocnemius muscle. Quantification of each sample was based on the entire intensities of each lane or region normalized to beta-tubulin. *p < .05
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
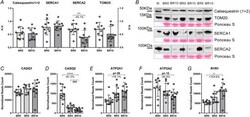
- Figure 7 Variations of gene and protein expression involved in Ca 2+ handling in response to 10-day bed rest Calsequestrin1-2, SERCA1, SERCA2 and TOM20 amount in muscle homogenates as obtained by western blot at baseline (BR0) and after 10 days of bed rest (BR10) ( A ). Representative western blot of CASQ1-2, SERCA1, SERCA2 and TOM20 ( B ). CASQ1 and CASQ2 gene expression (encoding for Calsequestrin1 and Calsequestrin2, respectively) ( C , D ), ATP2A1 and ATP2A2 gene expression (encoding for SERCA1 and SERCA2, respectively) ( E , F ); RYR1 gene expression (encoding for Ryanodine Receptor 1) ( G ). All RNA transcripts are reported as normalized read count at BR0, after 5 days of bed rest (BR5) and at BR10. Results shown as means +- SD, individual data represented as scatter plots. * P < 0.05 BR10 vs BR0, ** P < 0.01 BR10 vs BR0, **** P < 0.0001 BR10 vs BR0.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
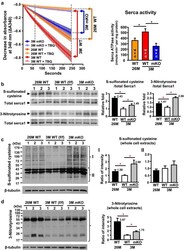
- 2 FIGURE Sarco(endo)plasmic reticulum Ca 2+ ATPase (SERCA) function is impaired and SERCA2a T-nitration is increased in soleus muscles of SOD2 +/- mice. (a) SERCA activity- p Ca curves in wild-type (WT) and SOD2 +/- mice over Ca 2+ concentrations ranging from p Ca 6.83-6.10, presented as % of V max . p Ca 50 values are embedded in the graph with 95% confidence intervals (CIs). (b) No differences in maximal SERCA activity ( mu mol/g of protein/min) were observed between genotypes. (c) Densitometric analysis and representative images of Western blots for SERCA2a, SERCA1a, and sarcolipin protein content as well as analyses and representative images of SERCA-specific T-nitration (d). All values are mean +- SEM and are presented relative to WT. * p < 0.05 ** p < 0.01, values above bars indicate p values using Student's t -test ( n = 5-8 per group).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
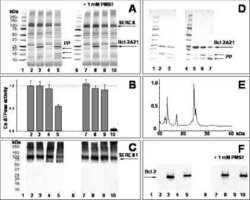
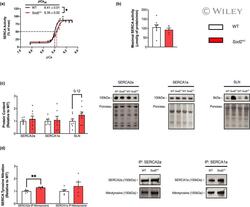
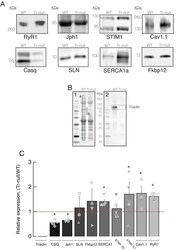
- 10.1371/journal.pone.0264146.g001 Fig 1 Effect of triadin ablation on the expression of couplon proteins. A , Western blot of proteins of interest from WT and Tr- total homogenates. Band signal mass was normalized to signal in the corresponding lane of the gel in B (boxed region a corrected for baseline level in b ). Details in Methods and []. B .1, Ponceau-stained proteins separated by PAGE. B .2, uncropped blot showing immunoreactivity of triadin antibody. C , distribution of results in Tr- samples as a ratio of the WT average. Different symbols identify values obtained from individual mice. Error bars represent SEM. Asterisks mark changes statistically significant at the 0.05 (*) level. Statistical parameters are listed in Table 1 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
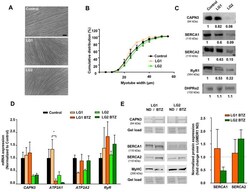
- FIGURE 2 Effect of UPS inhibition in myotubes from LGMDR1 patients. (A) Representative bright-field images showing immortalized human myotubes from a control and two LGMDR1 patients (LG1 and LG2) after 5 days in differentiation. Scale bar: 50 mum. (B) Cumulative distribution of myotube width from control, LG1 and LG2 myotubes. 90-170 myotubes analyzed from n = 3 independent experiments. Data are expressed as mean % +- SEM (two-way ANOVA test). (C) Western blot analysis of CAPN3, SERCA1, SERCA2, RyR1, and DHPRa2. Quantification of protein signals is shown below each blot, represented as fold change over control. (D) qPCR expression analysis of Ca 2+ -handling proteins in myotubes from control and LGMDR1 patients, treated or not with BTZ. Data are expressed as mean +- SEM. n = 3 independent experiments. * p < 0.05, One-way ANOVA post hoc Tukey's multiple comparisons test. (E) Representative western blot signals from LG1 and LG2 myotubes treated with 5 nM BTZ for 24 h after 5 days of differentiation and their quantification. Protein signals are normalized to total protein (gel load) and represented by fold change over non-treated LGMDR1 myotubes. Non-treated LGMDR1 SERCA expression levels are shown as a discontinuous line.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
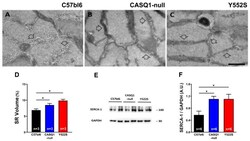
- Qualitative and quantitative EM of SR and WB analysis of SERCA-1 expression. ( A - C ) Representative EM images of EDL muscle fibers from WT (panel ( A )), CASQ1- (panel ( B )), and Y522S (panel ( C )) mice showing longitudinal SR cisternae appearance (empty arrows). ( D ) Quantitative analysis of the relative fiber volume occupied by the SR. ( E , F ) Representative immunoblots (panel ( E )) and relative band densities normalized to GAPDH levels of SERCA-1 expression (panel ( F )) in EDL muscle homogenates. Data are given as mean +- SEM (* p < 0.05), as evaluated by chi-squared test with Yates's correction for continuity (panel ( D )) or by one-way ANOVA followed by Tukey's post hoc test (panel ( F )). Scale bar ( A - C ), 1 um. n = number of mice.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
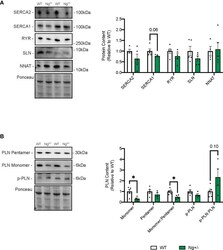
- Phospholamban is reduced in the soleus muscles of Ng +/- mice. Representative Western blots and their corresponding analyses for SERCA2a, SERCA1a, RYR, SLN and NNAT in the soleus of WT and Ng +/- mice (A) . Only reductions in SERCA1a content were observed, though this did not reach statistical significance. Characterizing PLN and its activation status showed reductions in monomeric PLN resulting in reductions in the monomer:pentamer ratio and statistically insignificant increases in the p-PLN : PLN ratio (B) . Molecular weight markers are included in the representative blots. * p < 0.05, values above bars indicate p values (n=4-5 per group).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
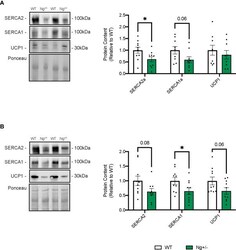
- Figure 5 SERCA content is reduced in iWAT and BAT from Ng +/- mice. Representative Western blots and corresponding analyses of SERCA2a, SERCA1a, and UCP1 from iWAT (A) and BAT (B) . In both depots, reductions in both SERCA isoforms were observed with no changes in UCP1. * p < 0.05, values above bars indicate p values (n=9-10 per group).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot