PA5-20872
antibody from Invitrogen Antibodies
Targeting: PRDM16
KIAA1675, KMT8F, MEL1, MGC166915, PFM13
Antibody data
- Antibody Data
- Antigen structure
- References [12]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [3]
- Other assay [12]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-20872 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- PRDM16 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA5-20872 can be used with blocking peptide PEP-0986.
- Reactivity
- Human, Mouse
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- 4°C
Submitted references Melatonin Regulates Differentiation of Sheep Brown Adipocyte Precursor Cells Via AMP-Activated Protein Kinase.
Imprinted lncRNA Dio3os preprograms intergenerational brown fat development and obesity resistance.
Fli1(+) cells transcriptional analysis reveals an Lmo2-Prdm16 axis in angiogenesis.
Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice.
Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice.
Novel discovery of Averrhoa bilimbi ethanolic leaf extract in the stimulation of brown fat differentiation program in combating diet-induced obesity.
Short-term thermoneutral housing alters glucose metabolism and markers of adipose tissue browning in response to a high-fat diet in lean mice.
Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRα(+) adipose progenitors.
Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis.
Alcohol intake aggravates adipose browning and muscle atrophy in cancer-associated cachexia.
Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring.
Apelin Enhances Brown Adipogenesis and Browning of White Adipocytes.
Gao XY, Deng BH, Li XR, Wang Y, Zhang JX, Hao XY, Zhao JX
Frontiers in veterinary science 2021;8:661773
Frontiers in veterinary science 2021;8:661773
Imprinted lncRNA Dio3os preprograms intergenerational brown fat development and obesity resistance.
Chen YT, Yang QY, Hu Y, Liu XD, de Avila JM, Zhu MJ, Nathanielsz PW, Du M
Nature communications 2021 Nov 25;12(1):6845
Nature communications 2021 Nov 25;12(1):6845
Fli1(+) cells transcriptional analysis reveals an Lmo2-Prdm16 axis in angiogenesis.
Matrone G, Xia B, Chen K, Denvir MA, Baker AH, Cooke JP
Proceedings of the National Academy of Sciences of the United States of America 2021 Aug 3;118(31)
Proceedings of the National Academy of Sciences of the United States of America 2021 Aug 3;118(31)
Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice.
Tian Q, Zhao J, Yang Q, Wang B, Deavila JM, Zhu MJ, Du M
Aging cell 2020 Jan;19(1):e13059
Aging cell 2020 Jan;19(1):e13059
Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice.
Son JS, Zhao L, Chen Y, Chen K, Chae SA, de Avila JM, Wang H, Zhu MJ, Jiang Z, Du M
Science advances 2020 Apr;6(16):eaaz0359
Science advances 2020 Apr;6(16):eaaz0359
Novel discovery of Averrhoa bilimbi ethanolic leaf extract in the stimulation of brown fat differentiation program in combating diet-induced obesity.
Lau WK, Noruddin NAA, Ariffin AH, Mahmud MZ, Noor MHM, Amanah A, Hamzah MF, Zafarina Z
BMC complementary and alternative medicine 2019 Sep 5;19(1):243
BMC complementary and alternative medicine 2019 Sep 5;19(1):243
Short-term thermoneutral housing alters glucose metabolism and markers of adipose tissue browning in response to a high-fat diet in lean mice.
Clayton ZS, McCurdy CE
American journal of physiology. Regulatory, integrative and comparative physiology 2018 Oct 1;315(4):R627-R637
American journal of physiology. Regulatory, integrative and comparative physiology 2018 Oct 1;315(4):R627-R637
Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRα(+) adipose progenitors.
Wang B, Fu X, Liang X, Deavila JM, Wang Z, Zhao L, Tian Q, Zhao J, Gomez NA, Trombetta SC, Zhu MJ, Du M
Cell discovery 2017;3:17036
Cell discovery 2017;3:17036
Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis.
Than A, Xu S, Li R, Leow MK, Sun L, Chen P
Signal transduction and targeted therapy 2017;2:17022
Signal transduction and targeted therapy 2017;2:17022
Alcohol intake aggravates adipose browning and muscle atrophy in cancer-associated cachexia.
Wang B, Zhang F, Zhang H, Wang Z, Ma YN, Zhu MJ, Du M
Oncotarget 2017 Nov 21;8(59):100411-100420
Oncotarget 2017 Nov 21;8(59):100411-100420
Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring.
Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, Du M
The Journal of physiology 2017 Mar 1;595(5):1547-1562
The Journal of physiology 2017 Mar 1;595(5):1547-1562
Apelin Enhances Brown Adipogenesis and Browning of White Adipocytes.
Than A, He HL, Chua SH, Xu D, Sun L, Leow MK, Chen P
The Journal of biological chemistry 2015 Jun 5;290(23):14679-91
The Journal of biological chemistry 2015 Jun 5;290(23):14679-91
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
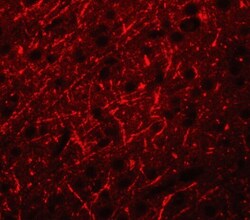
- Experimental details
- Immunofluorescence of PRDM16 in Human Brain cells with PRDM16 Polyclonal Antibody (Product # PA5-20872) at 20 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
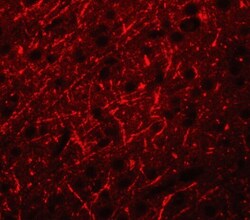
- Experimental details
- Immunofluorescence of PRDM16 in Human Brain cells with PRDM16 Polyclonal Antibody (Product # PA5-20872) at 20 µg/mL.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
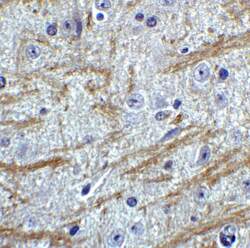
- Experimental details
- Immunohistochemistry of PRDM16 in mouse brain tissue with PRDM16 Polyclonal Antibody (Product # PA5-20872) at 2 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
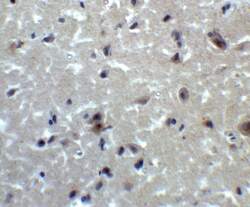
- Experimental details
- Immunohistochemistry of PRDM16 in human brain tissue with PRDM16 Polyclonal Antibody (Product # PA5-20872) at 2 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
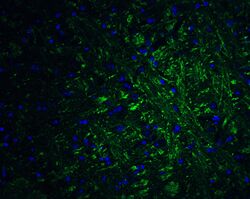
- Experimental details
- Immunofluorescence of PRDM16 in mouse brain tissue with PRDM16 Polyclonal Antibody (Product # PA5-20872) at 20 µg/mL. Green: PRDM16 Blue: DAPI staining
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
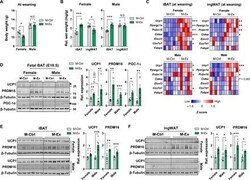
- Fig. 1 Maternal exercise intervention markedly induces the expression of thermogenic markers in iBAT and ingWAT of offspring at weaning. ( A and B ) Body weight (A) and relative wet weight of iBAT and ingWAT (B) normalized to body weight of female and male M-Ctrl and M-Ex offspring at weaning ( n = 6 mice per group). N.S., not significant. ( C ) Z scores of mRNA levels of different brown fat markers in the iBAT and ingWAT of M-Ctrl and M-Ex offspring at weaning ( n = 6 mice per group). Expression was normalized by Delta C t values. ( D ) Cropped Western blots of UCP1, PRDM16, and PGC-1alpha in the iBAT (beta-tubulin is the loading control) from the E18.5 fetuses of CON and EX maternal mice ( n = 6 fetuses per group). ( E and F ) Cropped Western blots of UCP1 and PRDM16 (beta-tubulin is the loading control) from iBAT (E) and ingWAT (F) isolated from female and male mice of M-Ctrl and M-Ex at weaning ( n = 6 mice per group). Data are mean +- SEM, and each dot represents one litter. * P < 0.05, ** P < 0.01, and *** P < 0.001 by two-tailed unpaired Student's t test (A to C, E, and F) or one-way analysis of variance (ANOVA) (D).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
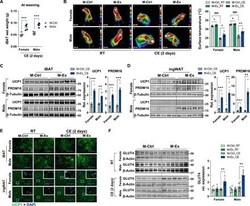
- Experimental details
- Fig. 2 Maternal exercise intervention facilitates thermogenesis in iBAT and ingWAT of offspring following CE. ( A ) iBAT wet weight of female and male M-Ctrl and M-Ex offspring by 2 days of CE ( n = 5 mice per group). ( B ) Representative thermographic images (left) and calculated averages of intrascapular temperature (right) of female and male M-Ctrl and M-Ex offspring at room temperature (RT) and 2 days of CE ( n = 6 litters for RT and n = 5 litters for CE). ( C and D ) Cropped Western blots of UCP1 and PRDM16 (beta-tubulin is the loading control) from iBAT (C) and ingWAT (D) of female and male M-Ctrl and M-Ex offspring followed by 2 days of CE ( n = 5 mice per group). ( E ) Representative images of UCP1 immunocytochemical staining of iBAT and ingWAT from M-Ctrl or M-Ex offspring at RT or CE. Scale bars, 100 mum. DAPI, 4',6-diamidino-2-phenylindole. ( F ) Cropped Western blots of GLUT4 in the iBAT (beta-actin was used as the loading control) isolated from M-Ctrl or M-Ex offspring at RT and after 2 days of CE ( n = 6 litters for RT and n = 5 litters for CE). Data are mean +- SEM, and each dot represents one litter. * P < 0.05, ** P < 0.01, and *** P < 0.001 in M-Ctrl versus M-Ex, and ### P < 0.001 in RT versus CE by two-tailed unpaired Student's t test (A, C, and D) or two-way ANOVA (B and F).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
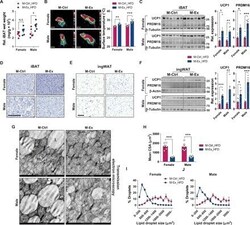
- Experimental details
- Fig. 4 Maternal exercise protects the impairment of thermogenic markers and formation in iBAT and ingWAT of the offspring challenged with HFD. ( A and B ) Relative iBAT wet weight (A) and representative thermographic images (left) and calculated averages of intrascapular temperature (right) (B) of female and male M-Ctrl and M-Ex offspring challenged with HFD ( n = 6). ( C and F ) Cropped Western blots of UCP1 and PRDM16 protein levels in the iBAT (C) and ingWAT (beta-tubulin or beta-actin were used as the loading control) (F) from the female and male M-Ctrl and M-Ex offspring challenged with HFD ( n = 6). ( D and G ) Hematoxylin and eosin (H&E) staining (D) and transmission electron microscopic images (G) of iBAT of female and male offspring challenged with HFD. Scale bars, 100 mum (D) and 1 mum (G); m.: mitochondria; l.: lipid droplets. ( E and H to J ) H&E staining (E), mean cross-sectional areas (CSAs) (H), and percent distribution of lipid droplets (I and J) in the ingWAT of female and male offspring challenged with HFD. Scale bar, 100 mum. Data are mean +- SEM, and each dot represents one litter. * P < 0.05, ** P < 0.01, and *** P < 0.001 in M-Ctrl versus M-Ex by two-tailed Student's t test (A to C, F, and H).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
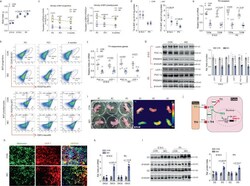
- Experimental details
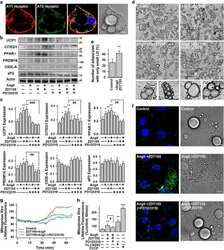
- Fig. 3 Maternal obesity (MO) reduces T3 concentration and thyroid hormone signaling, linking to impaired brown adipogenesis with a fetal origin. a Fetal mass in control and obesity dam (MO) at 22 degC ( n = 6). b Density of brown progenitors (solid circle) and preadipocytes (dash circle) in female offspring BAT at P0, 21 days (weaning), and 4 months of age. FACS sorted brown progenitors and preadipocytes in BAT-stromal vascular fractions using brown progenitor marker Lin: CD45-/PDGFRa+ (in black solid circle) and brown preadipocyte marker Lin: EBF2+/PDGFRa+ (dash black circle in right side quarter). c Quantified brown progenitors and preadipocytes in BAT of female offspring ( n = 5). Whisker of box plots shows means and individual values from minimum to maximum. d Thyroid hormone (TH) thyroxine (T4) and triiodothyronine (T3) concentrations were analyzed in fetal and neonatal BAT at embryonic days E18.5 ( n = 15) and P0 ( n = 17). e , f mRNA expression of TH receptors TRalpha and TRbeta ( e ), and TH responsive genes Ppargc1a and Ucp-1 ( f ) in fetal and neonatal BAT at E18.5 and P0 ( n = 6). mRNA expression was normalized to 18 S rRNA. g Immunoblotting measurements of PGC-1a, UCP-1, and PRDM16 protein contents in fetal (E18.5) and neonatal BAT ( n = 6). beta-tubulin was used as a loading control. h Immunostaining of mitochondrial (mito-tracker) and UCP-1 protein in neonatal BAT ( n = 4). Scale bar 100 um. i Thermal imaging of female neonates born to control and MO dam ( n = 1
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
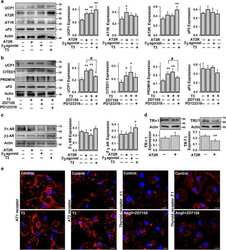
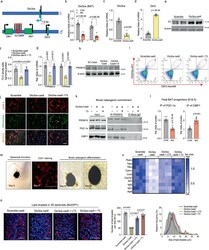
- Fig. 4 Dio3os suppression reduces T3 availability and PRDM16 activity, impairing brown adipogenesis in mouse embryonic fibroblasts and ex vivo organoids. a Diagram shows the Dlk1-Dio3 imprinting locus. Dio3os is a maternally imprinted long-coding RNA (LncRNA), located ~1.6 kb upstream of the paternal Dio3 gene. IG-DMR: imprinting control region with different DNA methylation. b mRNA expression of Dio3os in BAT of female fetuses and neonates at E18 and P0 ( n = 6). Expression was normalized to 18 S rRNA. c - n Pluripotent mouse embryonic fibroblasts (MEFs) were transfected with Cas9 nuclease, CRISPR-tracrRNA, scramble, or Dio3os crRNA (Dio3os-cas9). MEFs were induced brown adipocyte differentiation for 6 days. MEFs in Dio3os-cas9 were also treated with 50 n M T3 (Dio3os-cas9 + T3) during brown adipocyte induction. c Following 72 h transfection, mRNA expression of Dio3os was measured in MEFs. Expression was normalized to 18 S rRNA ( n = 4). d , e mRNA expression ( d ) and protein content of Dio3 ( e ) after 2 days of brown adipogenic commitment ( n = 4). f T3 concentration in differentiated brown adipocytes (2 days) ( n = 5). g mRNA expression of thyroid hormone receptors in differentiated brown adipocytes (2 days) ( n = 4). h After brown adipogenic commitment for 2 days, immunoblotting measurement of PRDM16 protein content. beta-actin was used as a loading control. i Population of committed brown preadipocytes (Lin: EBF2+/PDGFRa+) measured by flow cytometry sorting (FACS) ( n
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
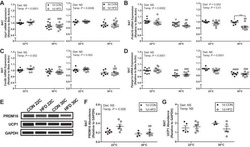
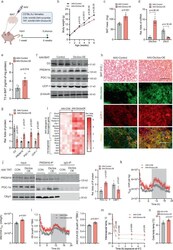
- Fig. 5 In vivo Dio3os expression activates brown adipogenesis and thermogenesis, improving energy expenditure and glucose tolerance. a C57BL/6 J female mice at 7 days of age ( n = 5) were injected with adeno-associated virus type 8 (AAV8)- Dio3os -CMV or AAV8-scramble-CMV (1.5 x 10 11 vg per mouse) into interscapular brown fat (BAT) at 22 degC. Mice were euthanized 5 weeks post-injection at 22 degC. b , c Body weight ( b ) and BAT mass ( c ) after AAV injections ( n = 5). d mRNA expression of Dio3os and Dio3 in interscapular BAT at 5 weeks post-injection ( n = 5). e T3 concentration ( e ) in BAT ( n = 5). f , g Immunoblotting measuring protein contents of D3, PRDM16, PGC-1a, and UCP-1 in BAT ( n = 5). beta-tubulin was as a loading control. h H&E staining and immunohistochemical staining of mitochondria (mito-tracker, green) and UCP-1 (red) in BAT ( n = 5). Scale bar 150 um (H&E) and 250 um (immunostaining). i Heatmap displaying mRNA expression of brown adipogenic and mitochondrial biogenic genes in BAT ( n = 5). j PRDM16 immunoprecipitation in measuring PGC-1a and CtBP1 complex in isolated BAT-SVFs. PRDM16, PGC-1a, and CtBP1 were detected by immunoblotting. IgG was used as a negative control ( n = 5). k , l Oxygen consumption ( k ) and energy expenditure ( l ) of mice at 5-week post AAV injections ( n = 5). Metabolic data were regressed to body mass according to guidelines of ANCOVA NIDDK MCCP tool. m , n Mice were exposed to 4 degC and surface body temperature ( m ) and core
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
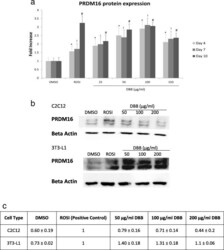
- Fig. 4 (a) Expression patterns of PRDM16 were responsive to treatment dose. 3 T3-L1 adipocytes were treated with either 0.5% DMSO, 1 muM ROSI or 25-150 mug/ml DBB for 4, 7 or 10 days respectively, followed by PRDM16 primary antibody labelling and FITC-conjugated secondary antibody conjugation. Image acquisition was performed using the IN Cell 2200 Analyzer and all fluorescent images were analysed using the IN Cell Developer software. * denotes statistically significance (ANOVA P < 0.05) as compared to DMSO (vehicle) after 4 days of treatment, |= denotes statistically significance (ANOVA P < 0.05) as compared to the vehicle after 7 days of treatment, and # indicates statistically significance (ANOVA P < 0.05) as compared to the vehicle after 10 days of treatment. (b) Western blot analysis was performed to detect the PRDM16 protein levels in 3 T3-L1 and C2C12 cells treated with 0.5% DMSO, 1 muM ROSI or 50-200 mug/ml DBB for 7 days. Beta actin was served as the loading control. (c) Quantification of PRDM16 protein normalised by beta actin and represented by fold expression
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
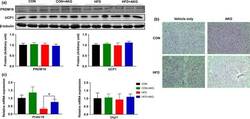
- Figure 4 Effect of alpha-ketoglutarate supplementation on metabolic activity of BAT. (a) PRDM16 and UCP1 contents in BAT analyzed by Western blot. (b) Representative H&E staining of BAT. (c) Prdm16 and Ucp1 mRNA levels in BAT analyzed by q-PCR. * p < .05 ( n = 6, mean +- SEM )
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry