Antibody data
- Antibody Data
- Antigen structure
- References [137]
- Comments [0]
- Validations
- Immunocytochemistry [13]
- Immunohistochemistry [1]
- Other assay [41]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-028 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- mtHSP70 Monoclonal Antibody (JG1)
- Antibody type
- Monoclonal
- Antigen
- Synthetic peptide
- Description
- MA3-028 detects mitochondrial heat shock protein 70 kDa (mtHSP70) from human, non-human primate, canine and mouse tissues. MA3-028 has been successfully used in Western blot, immunocytochemical, immunofluorescence, immunohistochemical (paraffin), and immunoprecipitation procedures. By Western blot, this antibody detects a single ~75 kDa band representing mtHSP70 from U2OS cell homogenate. Immunocytochemical staining of mtHSP70 in DAP.3 cells with MA3-028 results in a worm-like staining pattern, consistent with mitochondrial localization. Antibodies to this protein (and modification) were previously sold as part of a Thermo Scientific Cellomics High Content Screening Kit. This replacement antibody is now recommended for researchers who need an antibody for high content cell based assays. It has been thoroughly tested and validated for cellular immunofluorescence (IF) applications. Further optimization including the selection of the most appropriate fluorescent Dylight conjugated secondary antibody may have to be performed for your high content assay.
- Reactivity
- Human, Mouse, Canine
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- JG1
- Vial size
- 100 μL
- Concentration
- 1.07 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references UPR(mt) activation improves pathological alterations in cellular models of mitochondrial diseases.
Balanced mitochondrial and cytosolic translatomes underlie the biogenesis of human respiratory complexes.
OPA1 Modulates Mitochondrial Ca(2+) Uptake Through ER-Mitochondria Coupling.
His domain protein tyrosine phosphatase and Rabaptin-5 couple endo-lysosomal sorting of EGFR with endosomal maturation.
Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs.
The FUS gene is dual-coding with both proteins contributing to FUS-mediated toxicity.
PP2A Regulates Phosphorylation-Dependent Isomerization of Cytoplasmic and Mitochondrial-Associated ATR by Pin1 in DNA Damage Responses.
Mitochondrial RNA granules are fluid condensates positioned by membrane dynamics.
BioID-based proteomic analysis of the Bid interactome identifies novel proteins involved in cell-cycle-dependent apoptotic priming.
Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473.
β-Hydroxybutyrate Increases Exercise Capacity Associated with Changes in Mitochondrial Function in Skeletal Muscle.
Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration.
OSMR controls glioma stem cell respiration and confers resistance of glioblastoma to ionizing radiation.
Identification of calnexin as a diacylglycerol acyltransferase-2 interacting protein.
Hypoxia induces rapid, STAT3 and ROS dependent, mitochondrial translocation of RelA(p65) and IκBα.
Dysregulation of Mitochondrial Ca(2+) Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1.
Impairments in age-dependent ubiquitin proteostasis and structural integrity of selective neurons by uncoupling Ran GTPase from the Ran-binding domain 3 of Ranbp2 and identification of novel mitochondrial isoforms of ubiquitin-conjugating enzyme E2I (ubc9) and Ranbp2.
Neurolastin, a dynamin family GTPase, translocates to mitochondria upon neuronal stress and alters mitochondrial morphology in vivo.
Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation.
Mitochondrial fusion and Bid-mediated mitochondrial apoptosis are perturbed by alcohol with distinct dependence on its metabolism.
The Protein Coded by a Short Open Reading Frame, Not by the Annotated Coding Sequence, Is the Main Gene Product of the Dual-Coding Gene MIEF1.
Knockdown of HSPA9 induces TP53-dependent apoptosis in human hematopoietic progenitor cells.
Severe oxidative stress in an acute inflammatory demyelinating model in the rhesus monkey.
The Pseudouridine Synthase RPUSD4 Is an Essential Component of Mitochondrial RNA Granules.
Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2(high) Breast Cancer.
Drug Library Screening for the Identification of Ionophores That Correct the Mistrafficking Disorder Associated with Oxalosis Kidney Disease.
E4F1 controls a transcriptional program essential for pyruvate dehydrogenase activity.
Strategic Positioning and Biased Activity of the Mitochondrial Calcium Uniporter in Cardiac Muscle.
Diacylglycerol acyltransferase-2 and monoacylglycerol acyltransferase-2 are ubiquitinated proteins that are degraded by the 26S proteasome.
Human 'brite/beige' adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice.
Glucagon-like peptide-1 inhibits vascular smooth muscle cell dedifferentiation through mitochondrial dynamics regulation.
Constitutive Activation of PINK1 Protein Leads to Proteasome-mediated and Non-apoptotic Cell Death Independently of Mitochondrial Autophagy.
Overexpression of ErbB2 renders breast cancer cells susceptible to 3-BrPA through the increased dissociation of hexokinase II from mitochondrial outer membrane.
APC binds the Miro/Milton motor complex to stimulate transport of mitochondria to the plasma membrane.
FABP-1 gene ablation impacts brain endocannabinoid system in male mice.
MPC1-like Is a Placental Mammal-specific Mitochondrial Pyruvate Carrier Subunit Expressed in Postmeiotic Male Germ Cells.
Immunoregulatory Protein B7-H3 Reprograms Glucose Metabolism in Cancer Cells by ROS-Mediated Stabilization of HIF1α.
Nanocurcumin Prevents Hypoxia Induced Stress in Primary Human Ventricular Cardiomyocytes by Maintaining Mitochondrial Homeostasis.
The BARD1 BRCT domain contributes to p53 binding, cytoplasmic and mitochondrial localization, and apoptotic function.
Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis.
ATR Plays a Direct Antiapoptotic Role at Mitochondria, which Is Regulated by Prolyl Isomerase Pin1.
Centrosome-intrinsic mechanisms modulate centrosome integrity during fever.
Defining the phospho-adhesome through the phosphoproteomic analysis of integrin signalling.
Genetic Background is a Key Determinant of Glomerular Extracellular Matrix Composition and Organization.
Trim33 Binds and Silences a Class of Young Endogenous Retroviruses in the Mouse Testis; a Novel Component of the Arms Race between Retrotransposons and the Host Genome.
Effects of chronic cerebral hypoperfusion and low-dose progesterone treatment on apoptotic processes, expression and subcellular localization of key elements within Akt and Erk signaling pathways in rat hippocampus.
Matrine suppresses proliferation and induces apoptosis in human cholangiocarcinoma cells through suppression of JAK2/STAT3 signaling.
Reduced levels of Hspa9 attenuate Stat5 activation in mouse B cells.
Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells.
Diverse intracellular pathogens activate type III interferon expression from peroxisomes.
The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria.
Neurobeachin regulates neurotransmitter receptor trafficking to synapses.
Brain region- and sex-specific modulation of mitochondrial glucocorticoid receptor phosphorylation in fluoxetine treated stressed rats: effects on energy metabolism.
Role of p53 in cAMP/PKA pathway mediated apoptosis.
Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism.
Cytoplasmic sequestration of the tumor suppressor p53 by a heat shock protein 70 family member, mortalin, in human colorectal adenocarcinoma cell lines.
HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody.
Specific β-containing integrins exert differential control on proliferation and two-dimensional collective cell migration in mammary epithelial cells.
Fluoxetine affects hippocampal plasticity, apoptosis and depressive-like behavior of chronically isolated rats.
Syndecan-4 independently regulates multiple small GTPases to promote fibroblast migration during wound healing.
Beclin 1-independent autophagy contributes to apoptosis in cortical neurons.
An overlapping reading frame in the PRNP gene encodes a novel polypeptide distinct from the prion protein.
Knockdown of Hspa9, a del(5q31.2) gene, results in a decrease in hematopoietic progenitors in mice.
Dual function of protein kinase C (PKC) in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced manganese superoxide dismutase (MnSOD) expression: activation of CREB and FOXO3a by PKC-alpha phosphorylation and by PKC-mediated inactivation of Akt, respectively.
Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis.
A NH2 tau fragment targets neuronal mitochondria at AD synapses: possible implications for neurodegeneration.
Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis.
Chronic social isolation compromises the activity of both glutathione peroxidase and catalase in hippocampus of male wistar rats.
Role for X-linked Inhibitor of apoptosis protein upstream of mitochondrial permeabilization.
Amino terminal hydrophobic import signals target the p14(ARF) tumor suppressor to the mitochondria.
Oxidative stress causes reversible changes in mitochondrial permeability and structure.
Different expression of alpha and beta mitochondrial estrogen receptors in the aging rat brain: interaction with respiratory complex V.
Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6.
The role of phosphorylated glucocorticoid receptor in mitochondrial functions and apoptotic signalling in brain tissue of stressed Wistar rats.
Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK.
MDM4 (MDMX) localizes at the mitochondria and facilitates the p53-mediated intrinsic-apoptotic pathway.
Helix 3 is necessary and sufficient for prion protein's anti-Bax function.
HDAC inhibitor, valproic acid, induces p53-dependent radiosensitization of colon cancer cells.
Calpain 1 and Calpastatin expression is developmentally regulated in rat brain.
Chronic social isolation is related to both upregulation of plasticity genes and initiation of proapoptotic signaling in Wistar rat hippocampus.
Loss of anti-Bax function in Gerstmann-Sträussler-Scheinker syndrome-associated prion protein mutants.
Carboxy-Terminal Modulator Protein (CTMP) is a mitochondrial protein that sensitizes cells to apoptosis.
Mitochondrial Lon protease is a human stress protein.
Dual specificity phosphatases 18 and 21 target to opposing sides of the mitochondrial inner membrane.
Proteomics-based identification of autoantibody against heat shock protein 70 as a diagnostic marker in esophageal squamous cell carcinoma.
Targets of caspase-6 activity in human neurons and Alzheimer disease.
Bax targeting to mitochondria occurs via both tail anchor-dependent and -independent mechanisms.
The Mcf1 toxin induces apoptosis via the mitochondrial pathway and apoptosis is attenuated by mutation of the BH3-like domain.
Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels.
The cyclophilin-like domain of Ran-binding protein-2 modulates selectively the activity of the ubiquitin-proteasome system and protein biogenesis.
Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase.
The N-terminal conformation of Bax regulates cell commitment to apoptosis.
Defective retrotranslocation causes loss of anti-Bax function in human familial prion protein mutants.
CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice.
Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells.
Selective killing of cancer cells by leaf extract of Ashwagandha: identification of a tumor-inhibitory factor and the first molecular insights to its effect.
Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis.
Tid1 isoforms are mitochondrial DnaJ-like chaperones with unique carboxyl termini that determine cytosolic fate.
WT p53, but not tumor-derived mutants, bind to Bcl2 via the DNA binding domain and induce mitochondrial permeabilization.
Molecular morphology and toxicity of cytoplasmic prion protein aggregates in neuronal and non-neuronal cells.
Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2.
Viral and cellular oncogenes induce rapid mitochondrial translocation of p53 in primary epithelial and endothelial cells early in apoptosis.
Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production.
53BP2 induces apoptosis through the mitochondrial death pathway.
Further characterization of human DNA polymerase delta interacting protein 38.
Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells.
Tumor cell pseudopodial protrusions. Localized signaling domains coordinating cytoskeleton remodeling, cell adhesion, glycolysis, RNA translocation, and protein translation.
Human mitochondrial complex I assembles through the combination of evolutionary conserved modules: a framework to interpret complex I deficiencies.
Tid1, a cochaperone of the heat shock 70 protein and the mammalian counterpart of the Drosophila tumor suppressor l(2)tid, is critical for early embryonic development and cell survival.
Translocation of full-length Bid to mitochondria during anoikis.
A cryptic targeting signal induces isoform-specific localization of p46Shc to mitochondria.
Stress induces mitochondria-mediated apoptosis independent of SAPK/JNK activation in embryonic stem cells.
Evaluation of the anti-proliferative and anti-oxidative activities of leaf extract from in vivo and in vitro raised Ashwagandha.
Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss.
Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif.
In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation.
The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia.
Isolation and expression of a novel mitochondrial septin that interacts with CRMP/CRAM in the developing neurones.
Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone.
Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone.
Spatial and temporal changes in Bax subcellular localization during anoikis.
Dual localization of human DNA topoisomerase IIIalpha to mitochondria and nucleus.
Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex.
The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c.
Late activation of apoptotic pathways plays a negligible role in mediating the cytotoxic effects of discodermolide and epothilone B in non-small cell lung cancer cells.
ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter.
Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization.
Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator.
The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase.
The tripartite motif family identifies cell compartments.
A mouse homologue of the Drosophila tumor suppressor l(2)tid gene defines a novel Ras GTPase-activating protein (RasGAP)-binding protein.
Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum.
Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis.
TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions.
TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions.
Generation and characterization of monoclonal antibodies specific for members of the mammalian 70-kDa heat shock protein family.
Generation and characterization of monoclonal antibodies specific for members of the mammalian 70-kDa heat shock protein family.
Suárez-Rivero JM, Pastor-Maldonado CJ, Povea-Cabello S, Álvarez-Córdoba M, Villalón-García I, Talaverón-Rey M, Suárez-Carrillo A, Munuera-Cabeza M, Reche-López D, Cilleros-Holgado P, Piñero-Perez R, Sánchez-Alcázar JA
Orphanet journal of rare diseases 2022 May 17;17(1):204
Orphanet journal of rare diseases 2022 May 17;17(1):204
Balanced mitochondrial and cytosolic translatomes underlie the biogenesis of human respiratory complexes.
Soto I, Couvillion M, Hansen KG, McShane E, Moran JC, Barrientos A, Churchman LS
Genome biology 2022 Aug 9;23(1):170
Genome biology 2022 Aug 9;23(1):170
OPA1 Modulates Mitochondrial Ca(2+) Uptake Through ER-Mitochondria Coupling.
Cartes-Saavedra B, Macuada J, Lagos D, Arancibia D, Andrés ME, Yu-Wai-Man P, Hajnóczky G, Eisner V
Frontiers in cell and developmental biology 2021;9:774108
Frontiers in cell and developmental biology 2021;9:774108
His domain protein tyrosine phosphatase and Rabaptin-5 couple endo-lysosomal sorting of EGFR with endosomal maturation.
Parkinson G, Roboti P, Zhang L, Taylor S, Woodman P
Journal of cell science 2021 Nov 1;134(21)
Journal of cell science 2021 Nov 1;134(21)
Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs.
Todkar K, Chikhi L, Desjardins V, El-Mortada F, Pépin G, Germain M
Nature communications 2021 Mar 30;12(1):1971
Nature communications 2021 Mar 30;12(1):1971
The FUS gene is dual-coding with both proteins contributing to FUS-mediated toxicity.
Brunet MA, Jacques JF, Nassari S, Tyzack GE, McGoldrick P, Zinman L, Jean S, Robertson J, Patani R, Roucou X
EMBO reports 2021 Jan 7;22(1):e50640
EMBO reports 2021 Jan 7;22(1):e50640
PP2A Regulates Phosphorylation-Dependent Isomerization of Cytoplasmic and Mitochondrial-Associated ATR by Pin1 in DNA Damage Responses.
Makinwa Y, Cartwright BM, Musich PR, Li Z, Biswas H, Zou Y
Frontiers in cell and developmental biology 2020;8:813
Frontiers in cell and developmental biology 2020;8:813
Mitochondrial RNA granules are fluid condensates positioned by membrane dynamics.
Rey T, Zaganelli S, Cuillery E, Vartholomaiou E, Croisier M, Martinou JC, Manley S
Nature cell biology 2020 Oct;22(10):1180-1186
Nature cell biology 2020 Oct;22(10):1180-1186
BioID-based proteomic analysis of the Bid interactome identifies novel proteins involved in cell-cycle-dependent apoptotic priming.
Pedley R, King LE, Mallikarjun V, Wang P, Swift J, Brennan K, Gilmore AP
Cell death & disease 2020 Oct 16;11(10):872
Cell death & disease 2020 Oct 16;11(10):872
Human thermogenic adipocyte regulation by the long noncoding RNA LINC00473.
Tran KV, Brown EL, DeSouza T, Jespersen NZ, Nandrup-Bus C, Yang Q, Yang Z, Desai A, Min SY, Rojas-Rodriguez R, Lundh M, Feizi A, Willenbrock H, Larsen TJ, Severinsen MCK, Malka K, Mozzicato AM, Deshmukh AS, Emanuelli B, Pedersen BK, Fitzgibbons T, Scheele C, Corvera S, Nielsen S
Nature metabolism 2020 May;2(5):397-412
Nature metabolism 2020 May;2(5):397-412
β-Hydroxybutyrate Increases Exercise Capacity Associated with Changes in Mitochondrial Function in Skeletal Muscle.
Monsalves-Alvarez M, Morales PE, Castro-Sepulveda M, Sepulveda C, Rodriguez JM, Chiong M, Eisner V, Lavandero S, Troncoso R
Nutrients 2020 Jun 29;12(7)
Nutrients 2020 Jun 29;12(7)
Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration.
Zhou S, Zhang W, Cai G, Ding Y, Wei C, Li S, Yang Y, Qin J, Liu D, Zhang H, Shao X, Wang J, Wang H, Yang W, Wang H, Chen S, Hu P, Sun L
Cell research 2020 Dec;30(12):1063-1077
Cell research 2020 Dec;30(12):1063-1077
OSMR controls glioma stem cell respiration and confers resistance of glioblastoma to ionizing radiation.
Sharanek A, Burban A, Laaper M, Heckel E, Joyal JS, Soleimani VD, Jahani-Asl A
Nature communications 2020 Aug 17;11(1):4116
Nature communications 2020 Aug 17;11(1):4116
Identification of calnexin as a diacylglycerol acyltransferase-2 interacting protein.
Brandt C, McFie PJ, Vu H, Chumala P, Katselis GS, Stone SJ
PloS one 2019;14(1):e0210396
PloS one 2019;14(1):e0210396
Hypoxia induces rapid, STAT3 and ROS dependent, mitochondrial translocation of RelA(p65) and IκBα.
Ivanova IG, Perkins ND
Bioscience reports 2019 Sep 30;39(9)
Bioscience reports 2019 Sep 30;39(9)
Dysregulation of Mitochondrial Ca(2+) Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1.
Debattisti V, Horn A, Singh R, Seifert EL, Hogarth MW, Mazala DA, Huang KT, Horvath R, Jaiswal JK, Hajnóczky G
Cell reports 2019 Oct 29;29(5):1274-1286.e6
Cell reports 2019 Oct 29;29(5):1274-1286.e6
Impairments in age-dependent ubiquitin proteostasis and structural integrity of selective neurons by uncoupling Ran GTPase from the Ran-binding domain 3 of Ranbp2 and identification of novel mitochondrial isoforms of ubiquitin-conjugating enzyme E2I (ubc9) and Ranbp2.
Patil H, Yoon D, Bhowmick R, Cai Y, Cho KI, Ferreira PA
Small GTPases 2019 Mar;10(2):146-161
Small GTPases 2019 Mar;10(2):146-161
Neurolastin, a dynamin family GTPase, translocates to mitochondria upon neuronal stress and alters mitochondrial morphology in vivo.
Lomash RM, Petralia RS, Holtzclaw LA, Tsuda MC, Wang YX, Badger JD 2nd, Cameron HA, Youle RJ, Roche KW
The Journal of biological chemistry 2019 Jul 26;294(30):11498-11512
The Journal of biological chemistry 2019 Jul 26;294(30):11498-11512
Mitochondrial adaptation in human mesenchymal stem cells following ionizing radiation.
Patten DA, Ouellet M, Allan DS, Germain M, Baird SD, Harper ME, Richardson RB
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2019 Aug;33(8):9263-9278
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2019 Aug;33(8):9263-9278
Mitochondrial fusion and Bid-mediated mitochondrial apoptosis are perturbed by alcohol with distinct dependence on its metabolism.
Naghdi S, Slovinsky WS, Madesh M, Rubin E, Hajnóczky G
Cell death & disease 2018 Oct 9;9(10):1028
Cell death & disease 2018 Oct 9;9(10):1028
The Protein Coded by a Short Open Reading Frame, Not by the Annotated Coding Sequence, Is the Main Gene Product of the Dual-Coding Gene MIEF1.
Delcourt V, Brunelle M, Roy AV, Jacques JF, Salzet M, Fournier I, Roucou X
Molecular & cellular proteomics : MCP 2018 Dec;17(12):2402-2411
Molecular & cellular proteomics : MCP 2018 Dec;17(12):2402-2411
Knockdown of HSPA9 induces TP53-dependent apoptosis in human hematopoietic progenitor cells.
Liu T, Krysiak K, Shirai CL, Kim S, Shao J, Ndonwi M, Walter MJ
PloS one 2017;12(2):e0170470
PloS one 2017;12(2):e0170470
Severe oxidative stress in an acute inflammatory demyelinating model in the rhesus monkey.
Dunham J, van de Vis R, Bauer J, Wubben J, van Driel N, Laman JD, 't Hart BA, Kap YS
PloS one 2017;12(11):e0188013
PloS one 2017;12(11):e0188013
The Pseudouridine Synthase RPUSD4 Is an Essential Component of Mitochondrial RNA Granules.
Zaganelli S, Rebelo-Guiomar P, Maundrell K, Rozanska A, Pierredon S, Powell CA, Jourdain AA, Hulo N, Lightowlers RN, Chrzanowska-Lightowlers ZM, Minczuk M, Martinou JC
The Journal of biological chemistry 2017 Mar 17;292(11):4519-4532
The Journal of biological chemistry 2017 Mar 17;292(11):4519-4532
Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2(high) Breast Cancer.
Rohlenova K, Sachaphibulkij K, Stursa J, Bezawork-Geleta A, Blecha J, Endaya B, Werner L, Cerny J, Zobalova R, Goodwin J, Spacek T, Alizadeh Pesdar E, Yan B, Nguyen MN, Vondrusova M, Sobol M, Jezek P, Hozak P, Truksa J, Rohlena J, Dong LF, Neuzil J
Antioxidants & redox signaling 2017 Jan 10;26(2):84-103
Antioxidants & redox signaling 2017 Jan 10;26(2):84-103
Drug Library Screening for the Identification of Ionophores That Correct the Mistrafficking Disorder Associated with Oxalosis Kidney Disease.
Hou S, Madoux F, Scampavia L, Janovick JA, Conn PM, Spicer TP
SLAS discovery : advancing life sciences R & D 2017 Aug;22(7):887-896
SLAS discovery : advancing life sciences R & D 2017 Aug;22(7):887-896
E4F1 controls a transcriptional program essential for pyruvate dehydrogenase activity.
Lacroix M, Rodier G, Kirsh O, Houles T, Delpech H, Seyran B, Gayte L, Casas F, Pessemesse L, Heuillet M, Bellvert F, Portais JC, Berthet C, Bernex F, Brivet M, Boutron A, Le Cam L, Sardet C
Proceedings of the National Academy of Sciences of the United States of America 2016 Sep 27;113(39):10998-1003
Proceedings of the National Academy of Sciences of the United States of America 2016 Sep 27;113(39):10998-1003
Strategic Positioning and Biased Activity of the Mitochondrial Calcium Uniporter in Cardiac Muscle.
De La Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, Mishra J, Williams D, Finkel T, Murphy E, Joseph SK, Sheu SS, Csordás G
The Journal of biological chemistry 2016 Oct 28;291(44):23343-23362
The Journal of biological chemistry 2016 Oct 28;291(44):23343-23362
Diacylglycerol acyltransferase-2 and monoacylglycerol acyltransferase-2 are ubiquitinated proteins that are degraded by the 26S proteasome.
Brandt C, McFie PJ, Stone SJ
The Biochemical journal 2016 Oct 15;473(20):3621-3637
The Biochemical journal 2016 Oct 15;473(20):3621-3637
Human 'brite/beige' adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice.
Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh HL, Kim JK, Cooper MP, Fitzgibbons T, Brehm MA, Corvera S
Nature medicine 2016 Mar;22(3):312-8
Nature medicine 2016 Mar;22(3):312-8
Glucagon-like peptide-1 inhibits vascular smooth muscle cell dedifferentiation through mitochondrial dynamics regulation.
Torres G, Morales PE, García-Miguel M, Norambuena-Soto I, Cartes-Saavedra B, Vidal-Peña G, Moncada-Ruff D, Sanhueza-Olivares F, San Martín A, Chiong M
Biochemical pharmacology 2016 Mar 15;104:52-61
Biochemical pharmacology 2016 Mar 15;104:52-61
Constitutive Activation of PINK1 Protein Leads to Proteasome-mediated and Non-apoptotic Cell Death Independently of Mitochondrial Autophagy.
Akabane S, Matsuzaki K, Yamashita S, Arai K, Okatsu K, Kanki T, Matsuda N, Oka T
The Journal of biological chemistry 2016 Jul 29;291(31):16162-74
The Journal of biological chemistry 2016 Jul 29;291(31):16162-74
Overexpression of ErbB2 renders breast cancer cells susceptible to 3-BrPA through the increased dissociation of hexokinase II from mitochondrial outer membrane.
Gao S, Chen X, Jin H, Ren S, Liu Z, Fang X, Zhang G
Oncology letters 2016 Feb;11(2):1567-1573
Oncology letters 2016 Feb;11(2):1567-1573
APC binds the Miro/Milton motor complex to stimulate transport of mitochondria to the plasma membrane.
Mills KM, Brocardo MG, Henderson BR
Molecular biology of the cell 2016 Feb 1;27(3):466-82
Molecular biology of the cell 2016 Feb 1;27(3):466-82
FABP-1 gene ablation impacts brain endocannabinoid system in male mice.
Martin GG, Chung S, Landrock D, Landrock KK, Huang H, Dangott LJ, Peng X, Kaczocha M, Seeger DR, Murphy EJ, Golovko MY, Kier AB, Schroeder F
Journal of neurochemistry 2016 Aug;138(3):407-22
Journal of neurochemistry 2016 Aug;138(3):407-22
MPC1-like Is a Placental Mammal-specific Mitochondrial Pyruvate Carrier Subunit Expressed in Postmeiotic Male Germ Cells.
Vanderperre B, Cermakova K, Escoffier J, Kaba M, Bender T, Nef S, Martinou JC
The Journal of biological chemistry 2016 Aug 5;291(32):16448-61
The Journal of biological chemistry 2016 Aug 5;291(32):16448-61
Immunoregulatory Protein B7-H3 Reprograms Glucose Metabolism in Cancer Cells by ROS-Mediated Stabilization of HIF1α.
Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, Schmitt DC, Vu T, McClellan S, Lin Y, Lin W, Piazza GA, Fodstad O, Tan M
Cancer research 2016 Apr 15;76(8):2231-42
Cancer research 2016 Apr 15;76(8):2231-42
Nanocurcumin Prevents Hypoxia Induced Stress in Primary Human Ventricular Cardiomyocytes by Maintaining Mitochondrial Homeostasis.
Nehra S, Bhardwaj V, Ganju L, Saraswat D
PloS one 2015;10(9):e0139121
PloS one 2015;10(9):e0139121
The BARD1 BRCT domain contributes to p53 binding, cytoplasmic and mitochondrial localization, and apoptotic function.
Tembe V, Martino-Echarri E, Marzec KA, Mok MT, Brodie KM, Mills K, Lei Y, DeFazio A, Rizos H, Kettle E, Boadle R, Henderson BR
Cellular signalling 2015 Sep;27(9):1763-71
Cellular signalling 2015 Sep;27(9):1763-71
Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis.
Naghdi S, Várnai P, Hajnóczky G
Proceedings of the National Academy of Sciences of the United States of America 2015 Oct 13;112(41):E5590-9
Proceedings of the National Academy of Sciences of the United States of America 2015 Oct 13;112(41):E5590-9
ATR Plays a Direct Antiapoptotic Role at Mitochondria, which Is Regulated by Prolyl Isomerase Pin1.
Hilton BA, Li Z, Musich PR, Wang H, Cartwright BM, Serrano M, Zhou XZ, Lu KP, Zou Y
Molecular cell 2015 Oct 1;60(1):35-46
Molecular cell 2015 Oct 1;60(1):35-46
Centrosome-intrinsic mechanisms modulate centrosome integrity during fever.
Vertii A, Zimmerman W, Ivshina M, Doxsey S
Molecular biology of the cell 2015 Oct 1;26(19):3451-63
Molecular biology of the cell 2015 Oct 1;26(19):3451-63
Defining the phospho-adhesome through the phosphoproteomic analysis of integrin signalling.
Robertson J, Jacquemet G, Byron A, Jones MC, Warwood S, Selley JN, Knight D, Humphries JD, Humphries MJ
Nature communications 2015 Feb 13;6:6265
Nature communications 2015 Feb 13;6:6265
Genetic Background is a Key Determinant of Glomerular Extracellular Matrix Composition and Organization.
Randles MJ, Woolf AS, Huang JL, Byron A, Humphries JD, Price KL, Kolatsi-Joannou M, Collinson S, Denny T, Knight D, Mironov A, Starborg T, Korstanje R, Humphries MJ, Long DA, Lennon R
Journal of the American Society of Nephrology : JASN 2015 Dec;26(12):3021-34
Journal of the American Society of Nephrology : JASN 2015 Dec;26(12):3021-34
Trim33 Binds and Silences a Class of Young Endogenous Retroviruses in the Mouse Testis; a Novel Component of the Arms Race between Retrotransposons and the Host Genome.
Isbel L, Srivastava R, Oey H, Spurling A, Daxinger L, Puthalakath H, Whitelaw E
PLoS genetics 2015 Dec;11(12):e1005693
PLoS genetics 2015 Dec;11(12):e1005693
Effects of chronic cerebral hypoperfusion and low-dose progesterone treatment on apoptotic processes, expression and subcellular localization of key elements within Akt and Erk signaling pathways in rat hippocampus.
Stanojlović M, Guševac I, Grković I, Zlatković J, Mitrović N, Zarić M, Horvat A, Drakulić D
Neuroscience 2015 Dec 17;311:308-21
Neuroscience 2015 Dec 17;311:308-21
Matrine suppresses proliferation and induces apoptosis in human cholangiocarcinoma cells through suppression of JAK2/STAT3 signaling.
Yang N, Han F, Cui H, Huang J, Wang T, Zhou Y, Zhou J
Pharmacological reports : PR 2015 Apr;67(2):388-93
Pharmacological reports : PR 2015 Apr;67(2):388-93
Reduced levels of Hspa9 attenuate Stat5 activation in mouse B cells.
Krysiak K, Tibbitts JF, Shao J, Liu T, Ndonwi M, Walter MJ
Experimental hematology 2015 Apr;43(4):319-30.e10
Experimental hematology 2015 Apr;43(4):319-30.e10
Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells.
Hu Z, Zeng Q, Zhang B, Liu H, Wang W
Biochimie 2014 Dec;107 Pt B:257-62
Biochimie 2014 Dec;107 Pt B:257-62
Diverse intracellular pathogens activate type III interferon expression from peroxisomes.
Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, Boulant S, Gehrke L, Cossart P, Kagan JC
Nature immunology 2014 Aug;15(8):717-26
Nature immunology 2014 Aug;15(8):717-26
The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria.
Jin SM, Youle RJ
Autophagy 2013 Nov 1;9(11):1750-7
Autophagy 2013 Nov 1;9(11):1750-7
Neurobeachin regulates neurotransmitter receptor trafficking to synapses.
Nair R, Lauks J, Jung S, Cooke NE, de Wit H, Brose N, Kilimann MW, Verhage M, Rhee J
The Journal of cell biology 2013 Jan 7;200(1):61-80
The Journal of cell biology 2013 Jan 7;200(1):61-80
Brain region- and sex-specific modulation of mitochondrial glucocorticoid receptor phosphorylation in fluoxetine treated stressed rats: effects on energy metabolism.
Adzic M, Lukic I, Mitic M, Djordjevic J, Elaković I, Djordjevic A, Krstic-Demonacos M, Matić G, Radojcic M
Psychoneuroendocrinology 2013 Dec;38(12):2914-24
Psychoneuroendocrinology 2013 Dec;38(12):2914-24
Role of p53 in cAMP/PKA pathway mediated apoptosis.
Rahimi A, Lee YY, Abdella H, Doerflinger M, Gangoda L, Srivastava R, Xiao K, Ekert PG, Puthalakath H
Apoptosis : an international journal on programmed cell death 2013 Dec;18(12):1492-9
Apoptosis : an international journal on programmed cell death 2013 Dec;18(12):1492-9
Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism.
Ding Y, Liu Z, Desai S, Zhao Y, Liu H, Pannell LK, Yi H, Wright ER, Owen LB, Dean-Colomb W, Fodstad O, Lu J, LeDoux SP, Wilson GL, Tan M
Nature communications 2012;3:1271
Nature communications 2012;3:1271
Cytoplasmic sequestration of the tumor suppressor p53 by a heat shock protein 70 family member, mortalin, in human colorectal adenocarcinoma cell lines.
Gestl EE, Anne Böttger S
Biochemical and biophysical research communications 2012 Jun 29;423(2):411-6
Biochemical and biophysical research communications 2012 Jun 29;423(2):411-6
HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody.
Rinaldo C, Moncada A, Gradi A, Ciuffini L, D'Eliseo D, Siepi F, Prodosmo A, Giorgi A, Pierantoni GM, Trapasso F, Guarguaglini G, Bartolazzi A, Cundari E, Schininà ME, Fusco A, Soddu S
Molecular cell 2012 Jul 13;47(1):87-98
Molecular cell 2012 Jul 13;47(1):87-98
Specific β-containing integrins exert differential control on proliferation and two-dimensional collective cell migration in mammary epithelial cells.
Jeanes AI, Wang P, Moreno-Layseca P, Paul N, Cheung J, Tsang R, Akhtar N, Foster FM, Brennan K, Streuli CH
The Journal of biological chemistry 2012 Jul 13;287(29):24103-12
The Journal of biological chemistry 2012 Jul 13;287(29):24103-12
Fluoxetine affects hippocampal plasticity, apoptosis and depressive-like behavior of chronically isolated rats.
Djordjevic A, Djordjevic J, Elaković I, Adzic M, Matić G, Radojcic MB
Progress in neuro-psychopharmacology & biological psychiatry 2012 Jan 10;36(1):92-100
Progress in neuro-psychopharmacology & biological psychiatry 2012 Jan 10;36(1):92-100
Syndecan-4 independently regulates multiple small GTPases to promote fibroblast migration during wound healing.
Brooks R, Williamson R, Bass M
Small GTPases 2012 Apr-Jun;3(2):73-9
Small GTPases 2012 Apr-Jun;3(2):73-9
Beclin 1-independent autophagy contributes to apoptosis in cortical neurons.
Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J
Autophagy 2011 Oct;7(10):1115-31
Autophagy 2011 Oct;7(10):1115-31
An overlapping reading frame in the PRNP gene encodes a novel polypeptide distinct from the prion protein.
Vanderperre B, Staskevicius AB, Tremblay G, McCoy M, O'Neill MA, Cashman NR, Roucou X
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011 Jul;25(7):2373-86
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011 Jul;25(7):2373-86
Knockdown of Hspa9, a del(5q31.2) gene, results in a decrease in hematopoietic progenitors in mice.
Chen TH, Kambal A, Krysiak K, Walshauser MA, Raju G, Tibbitts JF, Walter MJ
Blood 2011 Feb 3;117(5):1530-9
Blood 2011 Feb 3;117(5):1530-9
Dual function of protein kinase C (PKC) in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced manganese superoxide dismutase (MnSOD) expression: activation of CREB and FOXO3a by PKC-alpha phosphorylation and by PKC-mediated inactivation of Akt, respectively.
Chung YW, Kim HK, Kim IY, Yim MB, Chock PB
The Journal of biological chemistry 2011 Aug 26;286(34):29681-90
The Journal of biological chemistry 2011 Aug 26;286(34):29681-90
Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis.
Chen X, Wong JY, Wong P, Radany EH
Molecular cancer research : MCR 2011 Apr;9(4):448-61
Molecular cancer research : MCR 2011 Apr;9(4):448-61
A NH2 tau fragment targets neuronal mitochondria at AD synapses: possible implications for neurodegeneration.
Amadoro G, Corsetti V, Stringaro A, Colone M, D'Aguanno S, Meli G, Ciotti M, Sancesario G, Cattaneo A, Bussani R, Mercanti D, Calissano P
Journal of Alzheimer's disease : JAD 2010;21(2):445-70
Journal of Alzheimer's disease : JAD 2010;21(2):445-70
Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis.
Trinh DL, Elwi AN, Kim SW
Oncotarget 2010 Oct;1(6):396-404
Oncotarget 2010 Oct;1(6):396-404
Chronic social isolation compromises the activity of both glutathione peroxidase and catalase in hippocampus of male wistar rats.
Djordjevic J, Djordjevic A, Adzic M, Radojcic MB
Cellular and molecular neurobiology 2010 Jul;30(5):693-700
Cellular and molecular neurobiology 2010 Jul;30(5):693-700
Role for X-linked Inhibitor of apoptosis protein upstream of mitochondrial permeabilization.
Owens TW, Foster FM, Valentijn A, Gilmore AP, Streuli CH
The Journal of biological chemistry 2010 Jan 8;285(2):1081-8
The Journal of biological chemistry 2010 Jan 8;285(2):1081-8
Amino terminal hydrophobic import signals target the p14(ARF) tumor suppressor to the mitochondria.
Irvine M, Philipsz S, Frausto M, Mijatov B, Gallagher SJ, Fung C, Becker TM, Kefford RF, Rizos H
Cell cycle (Georgetown, Tex.) 2010 Feb 15;9(4):829-39
Cell cycle (Georgetown, Tex.) 2010 Feb 15;9(4):829-39
Oxidative stress causes reversible changes in mitochondrial permeability and structure.
Cole NB, Daniels MP, Levine RL, Kim G
Experimental gerontology 2010 Aug;45(7-8):596-602
Experimental gerontology 2010 Aug;45(7-8):596-602
Different expression of alpha and beta mitochondrial estrogen receptors in the aging rat brain: interaction with respiratory complex V.
Alvarez-Delgado C, Mendoza-Rodríguez CA, Picazo O, Cerbón M
Experimental gerontology 2010 Aug;45(7-8):580-5
Experimental gerontology 2010 Aug;45(7-8):580-5
Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6.
Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ
Science signaling 2009 Sep 8;2(87):ra51
Science signaling 2009 Sep 8;2(87):ra51
The role of phosphorylated glucocorticoid receptor in mitochondrial functions and apoptotic signalling in brain tissue of stressed Wistar rats.
Adzic M, Djordjevic A, Demonacos C, Krstic-Demonacos M, Radojcic MB
The international journal of biochemistry & cell biology 2009 Nov;41(11):2181-8
The international journal of biochemistry & cell biology 2009 Nov;41(11):2181-8
Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK.
Owens TW, Valentijn AJ, Upton JP, Keeble J, Zhang L, Lindsay J, Zouq NK, Gilmore AP
Cell death and differentiation 2009 Nov;16(11):1551-62
Cell death and differentiation 2009 Nov;16(11):1551-62
MDM4 (MDMX) localizes at the mitochondria and facilitates the p53-mediated intrinsic-apoptotic pathway.
Mancini F, Di Conza G, Pellegrino M, Rinaldo C, Prodosmo A, Giglio S, D'Agnano I, Florenzano F, Felicioni L, Buttitta F, Marchetti A, Sacchi A, Pontecorvi A, Soddu S, Moretti F
The EMBO journal 2009 Jul 8;28(13):1926-39
The EMBO journal 2009 Jul 8;28(13):1926-39
Helix 3 is necessary and sufficient for prion protein's anti-Bax function.
Laroche-Pierre S, Jodoin J, LeBlanc AC
Journal of neurochemistry 2009 Feb;108(4):1019-31
Journal of neurochemistry 2009 Feb;108(4):1019-31
HDAC inhibitor, valproic acid, induces p53-dependent radiosensitization of colon cancer cells.
Chen X, Wong P, Radany E, Wong JY
Cancer biotherapy & radiopharmaceuticals 2009 Dec;24(6):689-99
Cancer biotherapy & radiopharmaceuticals 2009 Dec;24(6):689-99
Calpain 1 and Calpastatin expression is developmentally regulated in rat brain.
Li Y, Bondada V, Joshi A, Geddes JW
Experimental neurology 2009 Dec;220(2):316-9
Experimental neurology 2009 Dec;220(2):316-9
Chronic social isolation is related to both upregulation of plasticity genes and initiation of proapoptotic signaling in Wistar rat hippocampus.
Djordjevic A, Adzic M, Djordjevic J, Radojcic MB
Journal of neural transmission (Vienna, Austria : 1996) 2009 Dec;116(12):1579-89
Journal of neural transmission (Vienna, Austria : 1996) 2009 Dec;116(12):1579-89
Loss of anti-Bax function in Gerstmann-Sträussler-Scheinker syndrome-associated prion protein mutants.
Jodoin J, Misiewicz M, Makhijani P, Giannopoulos PN, Hammond J, Goodyer CG, LeBlanc AC
PloS one 2009 Aug 14;4(8):e6647
PloS one 2009 Aug 14;4(8):e6647
Carboxy-Terminal Modulator Protein (CTMP) is a mitochondrial protein that sensitizes cells to apoptosis.
Parcellier A, Tintignac LA, Zhuravleva E, Cron P, Schenk S, Bozulic L, Hemmings BA
Cellular signalling 2009 Apr;21(4):639-50
Cellular signalling 2009 Apr;21(4):639-50
Mitochondrial Lon protease is a human stress protein.
Ngo JK, Davies KJ
Free radical biology & medicine 2009 Apr 15;46(8):1042-8
Free radical biology & medicine 2009 Apr 15;46(8):1042-8
Dual specificity phosphatases 18 and 21 target to opposing sides of the mitochondrial inner membrane.
Rardin MJ, Wiley SE, Murphy AN, Pagliarini DJ, Dixon JE
The Journal of biological chemistry 2008 May 30;283(22):15440-50
The Journal of biological chemistry 2008 May 30;283(22):15440-50
Proteomics-based identification of autoantibody against heat shock protein 70 as a diagnostic marker in esophageal squamous cell carcinoma.
Fujita Y, Nakanishi T, Miyamoto Y, Hiramatsu M, Mabuchi H, Miyamoto A, Shimizu A, Takubo T, Tanigawa N
Cancer letters 2008 May 18;263(2):280-90
Cancer letters 2008 May 18;263(2):280-90
Targets of caspase-6 activity in human neurons and Alzheimer disease.
Klaiman G, Petzke TL, Hammond J, Leblanc AC
Molecular & cellular proteomics : MCP 2008 Aug;7(8):1541-55
Molecular & cellular proteomics : MCP 2008 Aug;7(8):1541-55
Bax targeting to mitochondria occurs via both tail anchor-dependent and -independent mechanisms.
Valentijn AJ, Upton JP, Bates N, Gilmore AP
Cell death and differentiation 2008 Aug;15(8):1243-54
Cell death and differentiation 2008 Aug;15(8):1243-54
The Mcf1 toxin induces apoptosis via the mitochondrial pathway and apoptosis is attenuated by mutation of the BH3-like domain.
Dowling AJ, Waterfield NR, Hares MC, Le Goff G, Streuli CH, ffrench-Constant RH
Cellular microbiology 2007 Oct;9(10):2470-84
Cellular microbiology 2007 Oct;9(10):2470-84
Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels.
Goetz JG, Genty H, St-Pierre P, Dang T, Joshi B, Sauvé R, Vogl W, Nabi IR
Journal of cell science 2007 Oct 15;120(Pt 20):3553-64
Journal of cell science 2007 Oct 15;120(Pt 20):3553-64
The cyclophilin-like domain of Ran-binding protein-2 modulates selectively the activity of the ubiquitin-proteasome system and protein biogenesis.
Yi H, Friedman JL, Ferreira PA
The Journal of biological chemistry 2007 Nov 30;282(48):34770-8
The Journal of biological chemistry 2007 Nov 30;282(48):34770-8
Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase.
Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E
The Journal of biological chemistry 2007 Nov 16;282(46):33583-33592
The Journal of biological chemistry 2007 Nov 16;282(46):33583-33592
The N-terminal conformation of Bax regulates cell commitment to apoptosis.
Upton JP, Valentijn AJ, Zhang L, Gilmore AP
Cell death and differentiation 2007 May;14(5):932-42
Cell death and differentiation 2007 May;14(5):932-42
Defective retrotranslocation causes loss of anti-Bax function in human familial prion protein mutants.
Jodoin J, Laroche-Pierre S, Goodyer CG, LeBlanc AC
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 May 9;27(19):5081-91
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 May 9;27(19):5081-91
CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice.
Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, Athar M
The Journal of clinical investigation 2007 Dec;117(12):3753-64
The Journal of clinical investigation 2007 Dec;117(12):3753-64
Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells.
Sirk D, Zhu Z, Wadia JS, Shulyakova N, Phan N, Fong J, Mills LR
Journal of neurochemistry 2007 Dec;103(5):1989-2003
Journal of neurochemistry 2007 Dec;103(5):1989-2003
Selective killing of cancer cells by leaf extract of Ashwagandha: identification of a tumor-inhibitory factor and the first molecular insights to its effect.
Widodo N, Kaur K, Shrestha BG, Takagi Y, Ishii T, Wadhwa R, Kaul SC
Clinical cancer research : an official journal of the American Association for Cancer Research 2007 Apr 1;13(7):2298-306
Clinical cancer research : an official journal of the American Association for Cancer Research 2007 Apr 1;13(7):2298-306
Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis.
Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS, Shore GC, McBride HM, Penninger JM, Slack RS
The EMBO journal 2006 Sep 6;25(17):4061-73
The EMBO journal 2006 Sep 6;25(17):4061-73
Tid1 isoforms are mitochondrial DnaJ-like chaperones with unique carboxyl termini that determine cytosolic fate.
Lu B, Garrido N, Spelbrink JN, Suzuki CK
The Journal of biological chemistry 2006 May 12;281(19):13150-8
The Journal of biological chemistry 2006 May 12;281(19):13150-8
WT p53, but not tumor-derived mutants, bind to Bcl2 via the DNA binding domain and induce mitochondrial permeabilization.
Tomita Y, Marchenko N, Erster S, Nemajerova A, Dehner A, Klein C, Pan H, Kessler H, Pancoska P, Moll UM
The Journal of biological chemistry 2006 Mar 31;281(13):8600-6
The Journal of biological chemistry 2006 Mar 31;281(13):8600-6
Molecular morphology and toxicity of cytoplasmic prion protein aggregates in neuronal and non-neuronal cells.
Grenier C, Bissonnette C, Volkov L, Roucou X
Journal of neurochemistry 2006 Jun;97(5):1456-66
Journal of neurochemistry 2006 Jun;97(5):1456-66
Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2.
Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E
Proceedings of the National Academy of Sciences of the United States of America 2006 Jul 5;103(27):10224-10229
Proceedings of the National Academy of Sciences of the United States of America 2006 Jul 5;103(27):10224-10229
Viral and cellular oncogenes induce rapid mitochondrial translocation of p53 in primary epithelial and endothelial cells early in apoptosis.
Nemajerova A, Wolff S, Petrenko O, Moll UM
FEBS letters 2005 Nov 7;579(27):6079-83
FEBS letters 2005 Nov 7;579(27):6079-83
Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production.
Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA
The Journal of biological chemistry 2005 Nov 11;280(45):37481-8
The Journal of biological chemistry 2005 Nov 11;280(45):37481-8
53BP2 induces apoptosis through the mitochondrial death pathway.
Kobayashi S, Kajino S, Takahashi N, Kanazawa S, Imai K, Hibi Y, Ohara H, Itoh M, Okamoto T
Genes to cells : devoted to molecular & cellular mechanisms 2005 Mar;10(3):253-60
Genes to cells : devoted to molecular & cellular mechanisms 2005 Mar;10(3):253-60
Further characterization of human DNA polymerase delta interacting protein 38.
Xie B, Li H, Wang Q, Xie S, Rahmeh A, Dai W, Lee MY
The Journal of biological chemistry 2005 Jun 10;280(23):22375-84
The Journal of biological chemistry 2005 Jun 10;280(23):22375-84
Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells.
Roucou X, Giannopoulos PN, Zhang Y, Jodoin J, Goodyer CG, LeBlanc A
Cell death and differentiation 2005 Jul;12(7):783-95
Cell death and differentiation 2005 Jul;12(7):783-95
Tumor cell pseudopodial protrusions. Localized signaling domains coordinating cytoskeleton remodeling, cell adhesion, glycolysis, RNA translocation, and protein translation.
Jia Z, Barbier L, Stuart H, Amraei M, Pelech S, Dennis JW, Metalnikov P, O'Donnell P, Nabi IR
The Journal of biological chemistry 2005 Aug 26;280(34):30564-73
The Journal of biological chemistry 2005 Aug 26;280(34):30564-73
Human mitochondrial complex I assembles through the combination of evolutionary conserved modules: a framework to interpret complex I deficiencies.
Ugalde C, Vogel R, Huijbens R, Van Den Heuvel B, Smeitink J, Nijtmans L
Human molecular genetics 2004 Oct 15;13(20):2461-72
Human molecular genetics 2004 Oct 15;13(20):2461-72
Tid1, a cochaperone of the heat shock 70 protein and the mammalian counterpart of the Drosophila tumor suppressor l(2)tid, is critical for early embryonic development and cell survival.
Lo JF, Hayashi M, Woo-Kim S, Tian B, Huang JF, Fearns C, Takayama S, Zapata JM, Yang Y, Lee JD
Molecular and cellular biology 2004 Mar;24(6):2226-36
Molecular and cellular biology 2004 Mar;24(6):2226-36
Translocation of full-length Bid to mitochondria during anoikis.
Valentijn AJ, Gilmore AP
The Journal of biological chemistry 2004 Jul 30;279(31):32848-57
The Journal of biological chemistry 2004 Jul 30;279(31):32848-57
A cryptic targeting signal induces isoform-specific localization of p46Shc to mitochondria.
Ventura A, Maccarana M, Raker VA, Pelicci PG
The Journal of biological chemistry 2004 Jan 16;279(3):2299-306
The Journal of biological chemistry 2004 Jan 16;279(3):2299-306
Stress induces mitochondria-mediated apoptosis independent of SAPK/JNK activation in embryonic stem cells.
Nishitai G, Shimizu N, Negishi T, Kishimoto H, Nakagawa K, Kitagawa D, Watanabe T, Momose H, Ohata S, Tanemura S, Asaka S, Kubota J, Saito R, Yoshida H, Mak TW, Wada T, Penninger JM, Azuma N, Nishina H, Katada T
The Journal of biological chemistry 2004 Jan 16;279(3):1621-6
The Journal of biological chemistry 2004 Jan 16;279(3):1621-6
Evaluation of the anti-proliferative and anti-oxidative activities of leaf extract from in vivo and in vitro raised Ashwagandha.
Kaur K, Rani G, Widodo N, Nagpal A, Taira K, Kaul SC, Wadhwa R
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2004 Dec;42(12):2015-20
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2004 Dec;42(12):2015-20
Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss.
Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL
Cancer research 2004 Dec 15;64(24):9217-24
Cancer research 2004 Dec 15;64(24):9217-24
Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif.
Schwer B, Ren S, Pietschmann T, Kartenbeck J, Kaehlcke K, Bartenschlager R, Yen TS, Ott M
Journal of virology 2004 Aug;78(15):7958-68
Journal of virology 2004 Aug;78(15):7958-68
In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation.
Erster S, Mihara M, Kim RH, Petrenko O, Moll UM
Molecular and cellular biology 2004 Aug;24(15):6728-41
Molecular and cellular biology 2004 Aug;24(15):6728-41
The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia.
Ribeiro CM, Paradiso AM, Livraghi A, Boucher RC
The Journal of general physiology 2003 Oct;122(4):377-87
The Journal of general physiology 2003 Oct;122(4):377-87
Isolation and expression of a novel mitochondrial septin that interacts with CRMP/CRAM in the developing neurones.
Takahashi S, Inatome R, Yamamura H, Yanagi S
Genes to cells : devoted to molecular & cellular mechanisms 2003 Feb;8(2):81-93
Genes to cells : devoted to molecular & cellular mechanisms 2003 Feb;8(2):81-93
Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone.
Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S
Molecular and cellular biology 2003 Feb;23(3):1085-94
Molecular and cellular biology 2003 Feb;23(3):1085-94
Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone.
Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S
Molecular and cellular biology 2003 Feb;23(3):1085-94
Molecular and cellular biology 2003 Feb;23(3):1085-94
Spatial and temporal changes in Bax subcellular localization during anoikis.
Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP
The Journal of cell biology 2003 Aug 18;162(4):599-612
The Journal of cell biology 2003 Aug 18;162(4):599-612
Dual localization of human DNA topoisomerase IIIalpha to mitochondria and nucleus.
Wang Y, Lyu YL, Wang JC
Proceedings of the National Academy of Sciences of the United States of America 2002 Sep 17;99(19):12114-9
Proceedings of the National Academy of Sciences of the United States of America 2002 Sep 17;99(19):12114-9
Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex.
Sargsyan E, Baryshev M, Szekely L, Sharipo A, Mkrtchian S
The Journal of biological chemistry 2002 May 10;277(19):17009-15
The Journal of biological chemistry 2002 May 10;277(19):17009-15
The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c.
Karpinich NO, Tafani M, Rothman RJ, Russo MA, Farber JL
The Journal of biological chemistry 2002 May 10;277(19):16547-52
The Journal of biological chemistry 2002 May 10;277(19):16547-52
Late activation of apoptotic pathways plays a negligible role in mediating the cytotoxic effects of discodermolide and epothilone B in non-small cell lung cancer cells.
Bröker LE, Huisman C, Ferreira CG, Rodriguez JA, Kruyt FA, Giaccone G
Cancer research 2002 Jul 15;62(14):4081-8
Cancer research 2002 Jul 15;62(14):4081-8
ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter.
Sharer JD, Shern JF, Van Valkenburgh H, Wallace DC, Kahn RA
Molecular biology of the cell 2002 Jan;13(1):71-83
Molecular biology of the cell 2002 Jan;13(1):71-83
Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization.
Chatellard-Causse C, Blot B, Cristina N, Torch S, Missotten M, Sadoul R
The Journal of biological chemistry 2002 Aug 9;277(32):29108-15
The Journal of biological chemistry 2002 Aug 9;277(32):29108-15
Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator.
Jungas T, Motta I, Duffieux F, Fanen P, Stoven V, Ojcius DM
The Journal of biological chemistry 2002 Aug 2;277(31):27912-8
The Journal of biological chemistry 2002 Aug 2;277(31):27912-8
The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase.
Schwer B, North BJ, Frye RA, Ott M, Verdin E
The Journal of cell biology 2002 Aug 19;158(4):647-57
The Journal of cell biology 2002 Aug 19;158(4):647-57
The tripartite motif family identifies cell compartments.
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A
The EMBO journal 2001 May 1;20(9):2140-51
The EMBO journal 2001 May 1;20(9):2140-51
A mouse homologue of the Drosophila tumor suppressor l(2)tid gene defines a novel Ras GTPase-activating protein (RasGAP)-binding protein.
Trentin GA, Yin X, Tahir S, Lhotak S, Farhang-Fallah J, Li Y, Rozakis-Adcock M
The Journal of biological chemistry 2001 Apr 20;276(16):13087-95
The Journal of biological chemistry 2001 Apr 20;276(16):13087-95
Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum.
Wang HJ, Guay G, Pogan L, Sauvé R, Nabi IR
The Journal of cell biology 2000 Sep 18;150(6):1489-98
The Journal of cell biology 2000 Sep 18;150(6):1489-98
Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC
The Journal of cell biology 1999 Mar 8;144(5):891-901
The Journal of cell biology 1999 Mar 8;144(5):891-901
TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions.
Syken J, De-Medina T, Münger K
Proceedings of the National Academy of Sciences of the United States of America 1999 Jul 20;96(15):8499-504
Proceedings of the National Academy of Sciences of the United States of America 1999 Jul 20;96(15):8499-504
TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions.
Syken J, De-Medina T, Münger K
Proceedings of the National Academy of Sciences of the United States of America 1999 Jul 20;96(15):8499-504
Proceedings of the National Academy of Sciences of the United States of America 1999 Jul 20;96(15):8499-504
Generation and characterization of monoclonal antibodies specific for members of the mammalian 70-kDa heat shock protein family.
Green JM, Gu L, Ifkovits C, Kaumaya PT, Conrad S, Pierce SK
Hybridoma 1995 Aug;14(4):347-54
Hybridoma 1995 Aug;14(4):347-54
Generation and characterization of monoclonal antibodies specific for members of the mammalian 70-kDa heat shock protein family.
Green JM, Gu L, Ifkovits C, Kaumaya PT, Conrad S, Pierce SK
Hybridoma 1995 Aug;14(4):347-54
Hybridoma 1995 Aug;14(4):347-54
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
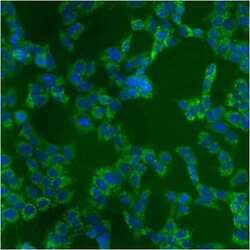
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in A549 Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
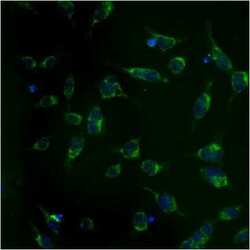
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in HMVEC Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
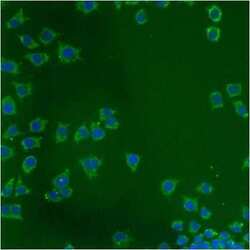
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in NS-1 Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in p19 Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
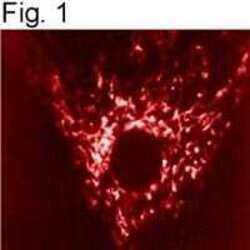
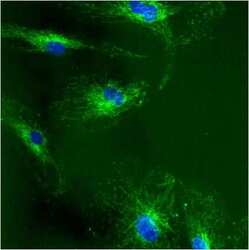
- Experimental details
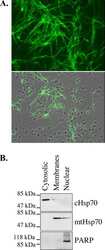
- Immunolocalization of mtHSP70 in human fibroblasts using Product # MA3-028.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
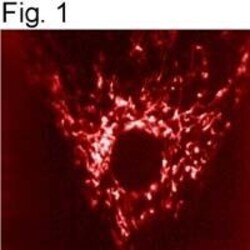
- Experimental details
- Immunolocalization of mtHSP70 in human fibroblasts using Product # MA3-028.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
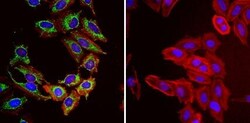
- Experimental details
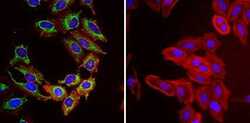
- Immunofluorescent analysis of Heat Shock Protein 70 (Hsp70, green) in HeLa cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with (left panel) or without (right panel) a Hsp70 monoclonal antibody (Product # MA3-028), at a dilution of 1:50 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat-anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with Dylight 554 phalloidin (Product # 21834), and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan or ToxInsight at 20X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in A549 Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
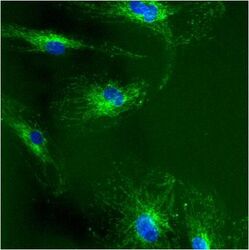
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in HMVEC Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
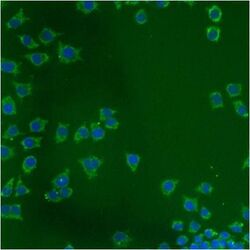
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in NS-1 Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
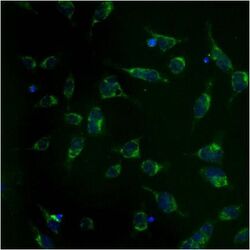
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 using anti-Heat Shock Protein 70 monoclonal antibody (Product # MA3-028) shows staining in p19 Cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunolocalization of mtHSP70 in human fibroblasts using Product # MA3-028.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of Heat Shock Protein 70 (Hsp70, green) in HeLa cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 10 minutes at room temperature and blocked with 1% Blocker BSA (Product # 37525) for 15 minutes at room temperature. Cells were probed with (left panel) or without (right panel) a Hsp70 monoclonal antibody (Product # MA3-028), at a dilution of 1:50 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat-anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:400 for 30 minutes at room temperature. F-Actin (red) was stained with Dylight 554 phalloidin (Product # 21834), and nuclei (blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ArrayScan or ToxInsight at 20X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
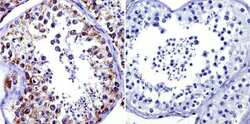
- Immunohistochemistry was performed on normal biopsies of deparaffinized human testis tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a Mouse Monoclonal Antibody recognizing Mitochondrial Heat Shock Protein 70 (Product # MA3-028) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
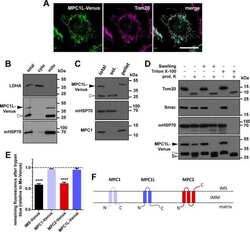
- Figure 1 Combined proteomic and phosphoproteomic analysis of isolated adhesion complexes. ( a ) Schematic workflow for the isolation and proteomic/phosphoproteomic analysis of adhesion complexes. Cells were allowed to spread on FN or, as a control, Tf and complexes were isolated by a combination of crosslinking, cell lysis and a high-pressure wash to remove cell bodies. Collected complexes were analysed using either a proteomic or phosphoproteomic workflow, after which the FN-specific proteins and phosphoproteins were identified by performing a subtractive comparison with controls. ( b ) Immunoblot analysis of complexes isolated from cells spread on FN and Tf, as well as the WCLs of cells spread on FN. M, MW markers (kDa; values displayed to the left of each blot). Dashed lines indicate where images have been cropped for display purposes. ( c ) A Venn diagram showing the overlap between the FN-specific proteins (left circle) and phosphoproteins (right circle) identified by proteomic and phosphoproteomic analyses of isolated complexes, respectively. In addition to the total number of proteins (black text), the number of adhesome proteins identified in each data set is also displayed (red text). To the right of the panel, all 19 adhesome components identified exclusively by the phosphoproteomic analysis are displayed. Proteins in bold text were not identified by any other proteomic analyses of isolated FN-induced adhesion complexes.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
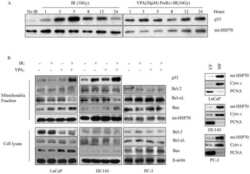
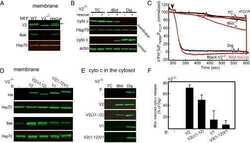
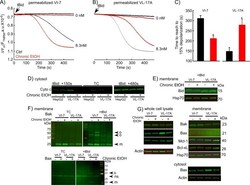
- Fig. 9 EtOH sensitizes HepG2 via increased Bak oligomerization but fails to sensitize VL-17A cells to tBid-induced OMMP. a , b Representative time-course recording of DeltaPsi m in permeabilized VI-7 a and VL-17A b cell suspension. Arrows show the addition of zero or 8.3 nM tBid. c Bar charts show the average of the time to reach to 15% of the depolarization upon treating the VI-7 and VL-17A cells with 8.3 nM tBid as described in Fig. 8a, b . (*: p < 0.05, n = 4). d Western blot using anti-cyto c antibody for the cytosolic fraction of the EtOH-exposed or non-exposed VI-7 and VL-17A cells. Permeabilized cells were treated with tBid for 150 s (left panel) or 450 s (right panel) and as described in Fig. 6e . TCs are the samples that were not treated with tBid. e Western blot using anti-Bid in the membrane fraction of EtOH-exposed and non-exposed VI-7 and VL-17A cells that were treated with 8.3 nM tBid. Hsp70 was used as loading control. f Western blot using anti-Bak (upper panels) and anti-Bax (lower panels) in the membrane fraction lysates of ethanol exposed and non-exposed VI-7 and VL-17A cells, which were treated with either zero (left panels) or 8.3 nM tBid (right panel). Membrane fractions were separated from cytosol and were treated with BMH (poly-linker) as described in Materials and methods. (O: oligomeric, ns: non-specific band, m: monomeric band) g Western blot of whole cell and membrane fraction lysates or cytosol fraction of the ethanol exposed
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
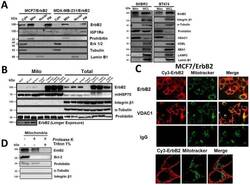
- Fig. 1 Localization of ErbB2 in mitochondria. (A) Cytosolic, nuclear, mitochondrial, and plasma membrane proteins were isolated and subjected to SDS-PAGE followed by probing with indicated antibodies. Two exogenous ErbB2 overexpressing breast cancer cell lines MCF7/ErbB2 and MDA-MB-231 (left), and two natural ErbB2-positive breast cancer cell lines SKBR3 and BT474 (right) were used for Western Blotting. VDAC1 and prohibitin were mitochondrial markers; Integrin beta1 and IGF1Ralpha were plasma membrane markers; alpha-Tubulin and ERK were cytoplasmic markers; KDEL was an ER marker; EEA1 was an early endosomes marker; Golgi complex was a marker for the detection of Golgi; LAMP2 was an lysosome marker and Lamin B1 was a nucleus marker. (B) MCF7 cells, mouse heart and liver tissues, and ErbB2 positive and negative breast cancer patient samples were analyzed by Western blotting. (C) Co-localization of ErbB2 and mitochondria. Mitochondria were stained with Mitotracker-Green in ErbB2 transfected MCF7 cells. The cells were fixed and incubated with antibodies against ErbB2 (mouse), followed by incubation of monoclonal mouse Anti-Cy3 antibody (red). Images were analyzed with Nikon NIS-Elements AR software. Green: mitochondria; Red: ErbB2; Yellow: Co-localization of ErbB2 and mitochondria. The lower panel contains images with a higher magnification. Scale bars: 20 mum. (D) Localization of ErbB2 inside mitochondria. Intact mitochondria of SKBR3 cells were isolated and treated with Proteas
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
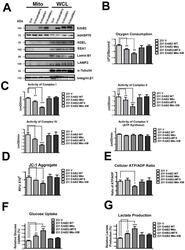
- Fig. 3 MtErbB2 reprograms cellular metabolism from oxidative phosphorylation toward glycolysis. (A) Mitochondrial and whole cell lysate of 231V, 231ErbB2WT, 231ErbB2Mito, and 231ErbB2DeltaMTS cells were isolated and analyzed by Western blotting. Protein amounts of each fraction loaded: mitochondria: 10 ug; whole cell lysate: 30 ug. Integrin beta1 was used as plasma membrane maker and loading control; mtHSP70 was a mitochondrial maker and loading control; alpha-Tubulin was a cytoplasm maker; KDEL was an ER marker; EEA1 was an early endosomes marker; Golgi Complex was a marker for the detection of Golgi; LAMP2 was an lysosome marker and Lamin B1 was a nucleus marker. (B) Oxygen consumption rates. Oxygen consumption rates of 231V, 231ErbB2WT, 231ErbB2Mito, 231ErbB2DeltaMTS and 231ErbB2MitoKM cells were measured. The oxygen consumption rate was calculated on the basis of the maximal rate of change in relative fluorescence units (DFU/second). (C) Activities of the mitochondrial electron transport chain complexes in 231V, 231ErbB2WT, 231ErbB2Mito, 231ErbB2DeltaMTS and 231ErbB2MitoKM cells. Activities are presented as milliunits of O.D. value per min and were normalized by the amounts of the mitochondrial proteins. (D) Mitochondrial membrane potential (DeltaPsim) of 231V, 231ErbB2WT, 231ErbB2Mito, 231ErbB2DeltaMTS and 231ErbB2MitoKM cells was detected using JC-1 staining. The aggregate form of JC-1 staining represents healthy mitochondria. (E) The cellular ATP/ADP ratio of 231V, 231
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
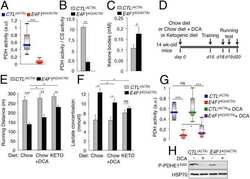
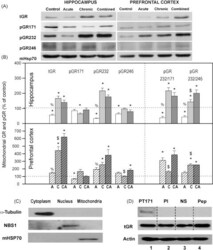
- Fig. 2 (A) Western blot experiments demonstrating the effects of acute immobilization chronic isolation and combined stress on the level of total glucocorticoid receptor (tGR) and its phosphoisoforms in mitochondria of hippocampus and prefrontal cortex. Mitochondrial lysates were resolved by SDS-PAGE and probed with antibodies against tGR, pGR171, pGR232, pGR246 or mHsp70 as a loading control. (B) Immunoreactivities of mitochondrial tGR, pGR171, pGR232, pGR246 (normalized to mHsp70) in hippocampus and prefrontal cortex. The ratios of pGR232/171 and pGR232/246 (normalized to GR and mHsp70) are expressed as mean +- SEM and presented as a percent of control (as described under Section 2 ). Asterisk indicates significant differences between treated groups: acute (A), chronic (C) and combined (CA) obtained from one-way ANOVA followed by Tukey post hoc test (* p < 0.05, stress vs. control; % p < 0.05, acute vs. combined; $ p < 0.05 chronic vs. combined). (C) The purity of subcellular fractions was assayed using specific antibodies against alpha-tubulin for cytoplasmic, NBS1 for nuclear, and mHsp70 for mitochondrial fraction. (D) Western blot of GR probed with antibody specific for its phosphorylated T171 isoform in the absence (lane 1) or presence (lane 4, PEP) of specific peptide used as antigen for this antibody. Membrane strips were also incubated with preimmune serum (lane 2, PI) or with non-specific IgG (lane 3, NS) (top panel). Blot was stripped of primary antibodies and prob
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
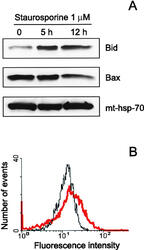
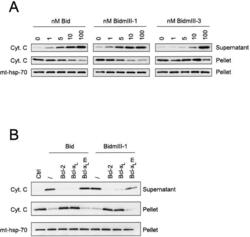
- Figure 4 Translocation of Bid to mitochondria during staurosporine-induced apoptosis of HeLa cells. (A) Mitochondria from HeLa cells before and after treatment with 1 muM staurosporine for 5 and 12 h were isolated on a sucrose gradient and analyzed for the presence of Bid and Bax by Western blotting. pAbs against full-length recombinant Bid (top) and to amino acids 1-21 of human Bax (middle) were used. Level of mt-hsp-70 (bottom) was used as a gel loading control. (B) Mitochondria from control HeLa cells (black curve) and HeLa cells treated with 1 muM staurosporine for 9 h (red curve) were isolated on a sucrose gradient, fixed, immunostained for Bid, and analyzed by flow cytometry.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 8 Bid-induced cytochrome c release from mitochondria. (A) Mitochondria from HeLa cells were isolated on a sucrose gradient, incubated for 15 min at 30degC with increasing concentrations of recombinant Bid as indicated. The incubation mix was centrifuged and the supernatants and pellets were analyzed for cytochrome c content by Western blot. mt-hsp-70 was used as a gel loading control. (B) Mitochondria from HeLa cells isolated on a sucrose gradient were incubated for 15 min at 30degC with 100 nM of wild-type Bid in the presence or absence of 1 muM of Bcl-2, Bcl-x L , or a mutant of Bcl-x L in which Gly 138 was replaced by an alanine (Bcl-x L m). Mitochondria were then centrifuged and the cytochrome c content of both the pellet and the supernatant of each sample was estimated by Western blotting. Equal loading of the mitochondrial pellet was confirmed with the antibody to mt-hsp-70.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
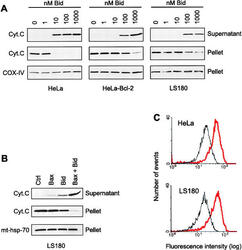
- Experimental details
- Figure 9 Involvement of Bax and Bcl-2 in the Bid-induced release of cytochrome c from mitochondria. (A) Mitochondria were isolated on a sucrose gradient from HeLa cells, Bcl-2-overexpressing HeLa cells, and from the Bax-deficient colon tumor cells LS180. They were incubated for 15 min at 30degC in the presence of increasing concentrations of recombinant Bid and the contents of cytochrome c of both the mitochondrial pellets and the supernatants were estimated by Western blot. Equal loading of the mitochondrial pellet was confirmed with the monoclonal anti-COX-IV antibody. (B) Mitochondria from LS180 cells were incubated for 15 min at 30degC with 5 muM recombinant Bax lacking 20 amino acids at the COOH terminus in the presence or absence of 50 nM recombinant Bid. Cytochrome c was detected by Western blot in the mitochondrial pellets and the supernatants. Equal loading of the mitochondrial pellet was confirmed with the antibody against mt-hsp-70. (C) Flow cytometric analysis of Bak immunostaining of mitochondria isolated from HeLa and LS180 cells. Mitochondria from both cell types were isolated on a sucrose gradient and incubated with 1 muM recombinant Bid for 15 min at 30degC before fixation, immunostaining with an antibody raised against NH 2 -terminal domain of Bak (amino acids 1-52; Calbiochem ), and FACS (r) analysis. Black curve: control. Red curve: 1 muM Bid.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
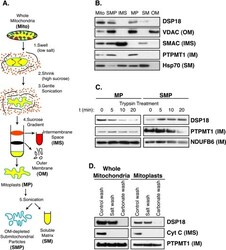
- Experimental details
- FIGURE 4. DSP18 is associated with the inner mitochondrial membrane facing the intermembrane space. A , diagram of mitochondrial subfractionation as described under ""Experimental Procedures."" B , 20 mug of each submitochondrial fraction (Fig. 4 A ) were separated by SDS-PAGE and immunoblotted with markers for OM (anti-VDAC), IMS (anti-SMAC), IM (anti-PTPMT1), and soluble matrix ( SM ; anti-Hsp70 - heat shock protein 70). C , 200 mug of MP and SMP were treated with 2.5 mug of trypsin in 100 mul of buffer for the indicated amounts of time. Samples were pelleted, washed, separated by SDS-PAGE, and immunoblotted with anti-DSP18 and anti-PTPMT1. The Complex 1 subunit NDUFB6 was not susceptible to trypsin digestion and was used as a loading control. D , 100 mug of osmotically swollen whole mitochondria and MP were washed with either control buffer, high salt (200 m m KCl, 2 m m HEPES, pH 7.2), or high pH (0.1 m Na 2 CO 3 , pH 11.5) buffer, repelleted, washed, and separated out by SDS-PAGE. Immunoblots were probed with antibodies against DSP18, cyt c, and PTPMT1.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
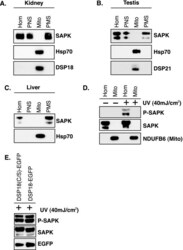
- Experimental details
- FIGURE 7. Endogenous SAPK/JNK is not localized to mitochondria. A , rat kidneys were isolated and homogenized ( HOM ), nuclei and unbroken cells were removed (post-nuclear supernatant (PNS)), Mito were removed, and the post-mitochondrial supernatant ( PMS ) was collected. Fractions were separated out by SDS-PAGE and immunoblotted with antibodies against both the p54 and p46 SAPK/JNK isoforms, mitochondrial marker protein Hsp70, and DSP18. B , fractions collected from testis tissue were separated out by SDS-PAGE and immunoblotted with antibodies against SAPK/JNK, Hsp70, and DSP21. C , fractions collected from rat liver tissue were separated out by SDS-PAGE and immunoblotted with antibodies against SAPK/JNK and Hsp70. D , HEK-293A cells were homogenized ( Hom ), and Mito were isolated by differential centrifugation after treatment with 40 mJ/cm 2 of UV radiation and compared with untreated controls. Fractions were separated out and immunoblotted with antibodies against phospho ( p )-SAPK/JNK (active), SAPK/JNK, and NDUFB6. E , HEK-293A cells transfected with DSP18-EGFP or a catalytic inactive mutant DSP18(C/S)-EGFP. After 24 h cells were treated with 40 mJ/cm 2 of UV radiation, and equal amounts of whole cell lysate were separated out by SDS-PAGE and analyzed for changes in SAPK/JNK phosphorylation.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
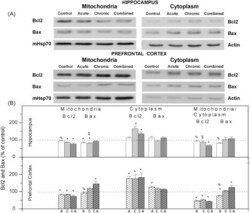
- Experimental details
- Fig. 4 (A) Western blot experiment demonstrating the effect of stress on mitochondrial and cytoplasmic Bcl2 and Bax levels in rat hippocampus and prefrontal cortex. (B) Immunorectivities of Bcl2 and Bax (normalized to mHsp70) in mitochondrial extracts and cytoplasm (normalized to beta-actin) of hippocampus or prefrontal cortex under acute (A), chronic (C) and combined (CA) stress and the ratio of mitochondrial to cytoplasmic Bcl2 and Bax are given as mean +- SEM, as a percent of control (as described under Section 2 ). Asterisk indicates significant differences obtained from one-way ANOVA followed by Tukey post hoc test (* p < 0.05, stress vs. control; % p < 0.05, acute vs. combined; $ p < 0.05 chronic vs. combined).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
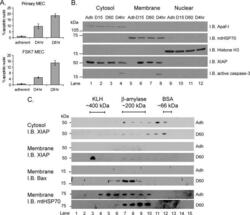
- Experimental details
- FIGURE 1. XIAP forms high molecular weight membrane-associated complexes in response to ECM withdrawal. A , primary mammary epithelial cells or FSK7 MECs were either left adherent or detached and plated onto poly(2-hydroxyethyl methacrylate)-coated dishes for 4 h ( D4hr ) or 8 h ( D8hr ). Cells were stained with DAPI, and apoptosis was scored by counting the percentage of nuclei with apoptotic morphology. Data represent the average of three independent experiments +- S.E. B , MECs were either left adherent ( Adh ) or detached for 15 min ( D15 ), 60 min ( D60 ), or 4 h ( D4hr ). Cells were then fractionated into cytosolic, membrane, and nuclear fractions and immunoblotted ( I.B. ) with the antibodies shown. Apaf-I, mtHSP70, and histone H3 were used as loading controls for each fraction, respectively. C , MECs were either left adherent ( Adh ) or detached for 60 min ( D60 ). Cytosolic or membrane fractions were separated by BN-PAGE (first dimension), then SDS-PAGE (second dimension), and immunoblotted with the antibodies shown. Keyhole limpet hemocyanin ( KLH ), beta-amylase, and bovine serum albumin ( BSA ) protein standards were used to calibrate the BN-PAGE. Membrane fractions were reprobed with anti-Bax and anti-mtHSP70.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
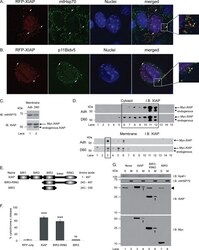
- Experimental details
- FIGURE 4. Membrane association of XIAP corresponds with ability to induce MOMP. A , MECs transiently expressing RFP-XIAP were stained with mitochondrial HSP70 ( green ). Note close association of RFP-XIAP with mtHSP70 ( arrows and enlarged view on right ). B , to confirm mitochondrial association of RFP-XIAP, cells were co-transfected with RFP-XIAP and a V5-epitope tagged form of Bid that constitutively localizes to mitochondria ( p11BidV5 ). C and D , MEF cells transiently expressing Myc-XIAP were either left adherent ( Adh ) or detached for 60 min ( D60 ) prior to fractionation. Samples were separated by SDS-PAGE only ( C ) or by BN-PAGE followed by SDS-PAGE ( D ) and immunoblotted for XIAP. Arrow and arrowhead indicate Myc-XIAP and endogenous XIAP, respectively. Note that the cytosolic Myc-XIAP was in a complex of the same size as the endogenous protein (compare lanes 6-12 D , upper panel , and Fig. 1 C , top panel ). E and F , MECs transiently expressing RFP, RFP-XIAP ( XIAP ), RFP-BIR3-RING, or RFP-BIR3 were scored for cytochrome c release. G , cytosolic and membrane fractions of MEF cells, untransfected or transiently expressing Myc-XIAP, Myc-BIR3-RING, or Myc-BIR3 were analyzed by immunoblotting for Myc and reprobing for XIAP. Arrowhead indicates endogenous XIAP. Arrows indicate transfected, membrane-localized XIAP fragments. Note that BIR3 is only present in the cytosolic fraction and that Myc-BIR3 is only detected by the Myc antibody because it does not contain the e
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
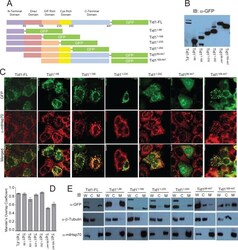
- Experimental details
- Figure 1 Cellular localization of Tid1 full length and its deletion mutants MCF-7 cells were transfected with Tid1 constructs to overexpress Tid1 full length or its domain deletion mutants (A) Schematic diagram of Tid1-EGFP full length or the domain deletion mutants (B) Immunoblotting analysis for Tid1 and its mutants. (C) Tid1-EGFP transfected cells labeled with anti-mtHsp70 were visualized by confocal microscope. (D) Mander's Overlap Co-efficient between the various Tid1 constructs and mtHsp70 as determined by Image J Intensity Correlation Analysis Software, with 1 being a high colocalization and 0 being low (n=4). Results are representative of a minimum of two independent experiments. (E) Cells were subjected to biochemical fractionation (W = whole cell extracts; C = cytosolic fraction; and M = mitochondrial fraction) followed by immunoblotting. Purity of the fractions was determined by immunoblotting with anti-mtHsp70 (mitochondrial) and anti-beta-tubulin (cytosolic) antibodies.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
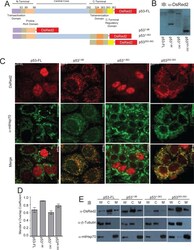
- Experimental details
- Figure 2 Cellular localization of p53 full length and its deletion mutants MCF-7 cells were transfected with p53 constructs to overexpress p53 full length or its domain deletion mutants (A) Schematic diagram of p53-DsRed2 full length or domain deletion mutants (B) Immunoblotting analysis for p53 and its mutants. (C) p53-DsRed2 transfected cells labeled with anti-mtHsp70 were visualized by confocal microscope. (D) Mander's Overlap Co-efficient between the various p53 constructs and mtHsp70 as determined as described in Fig. 1 D . (E) Cellular fraction were obtained as described in Fig. 1 E .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
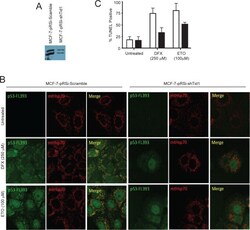
- Experimental details
- Figure 5 Suppression of Tid1 leads to loss of p53 at mitochondria and resistance to apoptosis following cellular stresses. MCF-7 cells stably expressing non-silencing shRNA (shScram) or shRNA directed against Tid1 (shTid1) were incubated in the presence or absence of 250 muM DFX or 100 muM for ETO 6 hr. (A) Tid1 expression in MCF-7 cells stably expressing shScram or shTid1. (B) Cells labeled with anti-p53 antibodies (green) and anti-mtHsp70 antibodies (red) were visualized by confocal microscopy. (C) Apoptotic cells were detected by in situ TUNEL assay.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
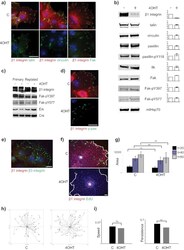
- Experimental details
- FIGURE 3. beta1-Integrin- MECs contain functional adhesomes and show collective two-dimensional cell migration. a , beta1 fx/fx ;CreER TM MECs treated with 4OHT or untreated controls ( C ) were cultured on collagen-I coated coverslips, fixed 3 days after isolation, and stained for beta1-integrin ( red ) and talin, vinculin, or Fak ( green ). Bar : 20 mum. b , immunoblotting of lysates from untreated and beta1-integrin-deleted (4OHT) MECs cultured for 3 days and probed for the indicated antigens. Mitochondrial Hsp70 ( mtHsp70 ) was used a loading control. The intensity of the bands was quantified using the Odyssey system, and the level of signal in the 4OHT-treated samples is plotted relative to untreated. Error bars = S.E. c , immunoblotting of lysates from CreER TM -only MECs replated onto collagen I. d , immunofluorescence staining of paxillin-Tyr(P)-31 ( p-pax ) in control and 4OHT-treated MECs. Bar : 20 mum. e , beta1- MECs stained with beta1- and beta3-integrin antibodies. Bar : 20 mum. f , control ( C ) and beta1- (4OHT) acini were plated onto two-dimensional collagen I-coated plates. The acini were allowed to attach, and the cells migrated onto the culture surface. At the end of the experiment, the cells were stained for EdU and beta1-integrin. Note the absence of EdU incorporation in the beta1- cells. Asterisks indicate location of acini from which the cells emigrated. White dotted line indicates migration front. Bar : 40 mum. g , acini were treated wi
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
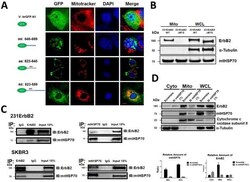
- Experimental details
- Fig. 2 Analysis of the mechanisms of mtErbB2 mitochondrial localization. (A) MCF7 breast cancer cells were transfected with plasmids encoding either GFP alone, or GFP fused ErbB2 fragments GFP-646-689, GFP-623-645 or GFP-623-689. Cells were cultured and the florescent imaging was done as described under the ""Methods."" Scale bars: 20 mum. (B) MDA-MB-231 cells were transfected with wild-type ErbB2 and ErbB2DeltaMTS vectors and mitochondrial proteins were extracted. ErbB2 expression was measured by Western blotting analysis; alpha-Tubulin and mtHSP70 were loading controls. (C) Mitochondrial proteins were isolated from MDA-MB-231ErbB2 cells and immunoprecipitated with ErbB2 antibody. The immunoprecipitates were probed with anti-mtHSP70 and anti-ErbB2 (top left). Mitochondrial proteins from the same cells were precipitated with mtHSP70 antibody and the immunoprecipitates were probed with anti-mtHSP70 and anti-ErbB2 (top right). IgG was used as a negative control. Isolated mitochondrial proteins (input) were loaded as a positive control. Similar results were obtained using another breast cancer cell line, SKBR3 (bottom). (D) siRNA specific to mtHSP70 was transfected into MDA-MB-231ErbB2 cells. The cytoplasmic fraction (Cyto), mitochondrial fraction (Mito) and whole cell lysate (WCL) were separated for western blotting analysis (left). Cytochrome c oxidase subunit II and alpha-Tubulin were makers and loading controls for the mitochondrial fraction and the cytoplasmic fraction, res
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
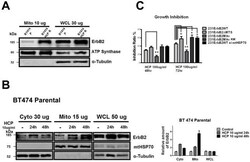
- Experimental details
- Fig. 5 Translocation of ErbB2 into mitochondria contributes to trastuzumab resistance. (A) mtErbB2 is elevated in trastuzumab-resistant cancer cells. Mitochondrial proteins and whole cell lysates of BT474 and BT474 trastuzumab-resistant cells (BT474 HCP R) were isolated and analyzed by Western blotting. ATP Synthase and alpha-Tubulin were used as loading controls. (B) BT474 cells were treated with trastuzumab (HCP) at 10 ug/ml for 24 h and 48 h followed by the separation of cytosolic and mitochondrial fractions. Proteins from the cytoplasm, mitochondria and whole cell lysates were loaded onto gels and analyzed by Western blotting. mtHSP70 and alpha-Tubulin were used as loading controls (left). The relative protein amount of ErbB2 in the cellular fractions was calculated by detecting the intensity of the protein bands followed by normalization with loading controls (right). The experiments were repeated for three times. (C) mtErbB2-overexpressing cells are more resistant to trastuzumab. 231ErbB2WT, 231ErbB2Mito, 231ErbB2DeltaMTS, 231ErbB2MitoKM and 231ErbB2WT cells transfected by siRNA to mtHSP70 were treated with trastuzumab at 100 ug/ml for 48 h and 72 h. The cell growth inhibition ratios were detected by a CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit. Columns, mean of three independent experiments; bars, SE. *, P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
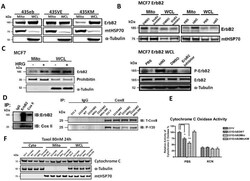
- Experimental details
- Fig. 6 Translocation of ErbB2 into mitochondria is kinase dependent. (A) The mitochondrial fractions and whole cell lysates were isolated and Western blotting was performed on MDA-MB-435ErbB2 (435eb), MDA-MB-435ErbB2V695E (435VE) and MDA-MB-435ErbB2K753M (435KM) cells. MtHSP70 and alpha-Tubulin were loading controls. (B) Mitochondrial fractions and whole cell lysates were isolated and Western Blotting was performed to detect mtErbB2 in mitochondria following the treatment of MCF7ErbB2 cells with ErbB2 inhibitor AG825 at 10 uM or control (DMSO) for 24 h; and with Heregulin beta1 (HRG) at 10 ng/ml or control (PBS) for 24 h (upper). Whole cell lysates were subjected to Western Blotting to examine the phosphorylation status of ErbB2 and the total ErbB2. alpha-Tubulin was loading control (lower). (C) MCF7 cells were treated with and without HRG at 10 ng/ml for 24 h. The mitochondrial fraction and the whole cell lysate were isolated for Western blotting. Prohibitin and alpha-Tubulin were loading controls. (D) Left, mitochondrial proteins were isolated from SKBR3 cells and immunoprecipitated with anti-ErbB2 or anti-Cox II antibodies. The immunoprecipitates were analyzed by Western blotting with anti-ErbB2 and anti-Cox II antibodies. IgG was negative control. Right, mitochondrial proteins were isolated from 231V, 231ErbB2WT, 231ErbB2Mito and 231ErbB2MitoKM cells and immunoprecipited with Cox II antibody or negative control IgG. Samples were loaded on a SDS-PAGE followed by Western Bl
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 6. Nbea localization in WT neurons. (A) Somatic Nbea concentrates near the ERGIC and Golgi complex. WT hippocampal neurons (E18) were cotransfected by a calcium phosphate precipitation method at DIV10-11 with plasmids encoding ERGIC53-GFP (ERGIC53) and mCherry (not depicted in the merge). At DIV14, cells were fixed and stained for endogenous Nbea with a rabbit polyclonal antibody (green) and for the markers indicated (ER marker KDEL, cis-Golgi marker GM130, and trans-Golgi marker TGN38 or Vti1A; dendritic marker MAP2 not depicted in the merge). All experiments were performed on mouse neurons, except for the analysis of TGN38, which was performed on rat neurons because of the lack of reactivity of the corresponding antibody in mouse cells. (B) Quantification of the colocalization of somatic Nbea with the different indicated markers using Pearson's correlation coefficient (PCC) and Manders' overlap coefficient (MOC). The numbers of cells analyzed are given in the histogram bars. (C) Dendritic Nbea localization. Cultured WT neurons were immunostained at DIV14 for Nbea, AnkyrinB, and MAP2. (D) Punctate localization pattern of Nbea in dendrites. Detail of a dendrite of a WT neuron costained for MAP2 (left) and Nbea (right). (E) Nbea-positive puncta show partial overlap with recycling endosomal and postsynaptic markers but low overlap with presynaptic and lysosomal markers. (top three images) DIV15 WT hippocampal neurons (cultured form E18 embryos) were transfected
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 5: Centrosome degradation is specific. (A) Mitochondria are not degraded during HS. Maximum projections from confocal microscopy images show representative images of cntrl and HS cells immunostained with ATP5A (left; bar,10 mum) and mitochondrial Hsp70 (green) and gamma- tubulin (red); middle and right, inset, centrosomes (bar, 10 mum). (B) Kinetochores are not degraded during HS. Maximum projections from confocal microscopy images show representative images of prometaphase cells from cntrl and HS cells immunostained with CenpE (green) and CREST (red); bar 10 mum. (C) Semiquantitative analysis of integrated intensity of the midbody marker RacGap (x10 5 arbitrary units, mean +- SEM) demonstrates no significant difference in signal intensity in HS cells. (D) Semiquantitative analysis of integrated intensity of the midbody marker mitotic kinesin-like protein (x10 5 arbitrary units, mean +- SEM) demonstrates no significant difference in signal intensity in HS cells. (E) Maximum projections from confocal microscopy images show representative images of cntrl and HS cells immunostained with Sept7 (red, decorates midbody, MB, and centrosomes, Cen) and Centrin2 (green, as centrosome marker); bar, 5 mum. HS results in loss of Septin7 from centrosomes but not from midbodies in the same cell. (F) Disruption of Septin7 signal after HS is shown at the centrosome but not at the midbody by semiquantitative analysis of integrated intensity (x10 5 arbitrary units, mean +- SEM).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
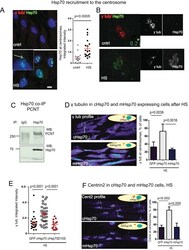
- Experimental details
- FIGURE 6: The centrosome is a substrate organelle for molecular chaperone Hsp70. (See also Supplemental Figures S1-S3.) (A) Left, images of maximum projections from cntrl and HS cells as indicated show that upon HS, Hsp70 accumulates at the centrosome and in the nucleus (inset, arrow points at the centrosome); bar, 10 mum. Semiquantitative analysis of integrated intensity (x10 5 arbitrary units) of centrosomal Hsp70 before and after HS (15-20 centrosomes/sample, mean +- SEM). (B) Superresolution images (OMX) demonstrate loss of gamma-tubulin (red) from HS cells and recruitment of Hsp70 (green) as an outer layer in stressed cells; bar, 0.2 mum. (C) Immunoprecipitation (IP) of Hsp70 pulls down PCNT. Immunoglobulin G (IgG), control. (D) Centrosome-targeted Hsp70 (cHsp70) protects gamma-tubulin from HS, whereas membrane-targeted Hsp70 (mHsp70) does not. Data are shown as semiquantitative profiles of confocal microscopy images. Right, percentage of cells with centrosomal gamma-tubulin after HS in stably expressing centrosome-(cHsp70) or membrane-(mHsp70) targeted Hsp70-expressing cells (four experiments, 500-600 cells/sample, mean +- SD, one-way analysis of variance [ANOVA] combined with Tukey's multiple comparison test). (E) cHsp70, but not the chaperone-negative mutant cHsp70D10S, protects gamma-tubulin signal at the centrosome; semiquantitative analysis of integrated gamma-tubulin signal intensity (x10 5 arbitrary units, mean +- SEM). (F) cHsp70 protects centrosomal Centri
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
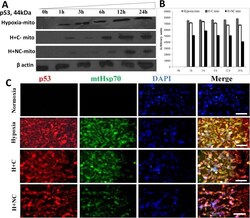
- Experimental details
- Fig 7 Nanocurcumin prevents hypoxia induced mitochondrial translocation of p53 in HVCM cells: Translocation of p53 in mitochondria (mito) in HVCM cells was time-dependent and initiated within1 h of hypoxia. Nanocurcumin prevented p53 translocation to mitochondria in HVCM cells under hypoxia better than curcumin (A, B). Also, enhancement in levels of p53 (Red) and mtHsp70 (Green) was observed after hypoxia insult in HVCM cells after 24 h (C). Nanocurcumin treatment down-regulated p53 and mtHsp70 levels better than curcumin treated cells under hypoxia. Bar represents 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
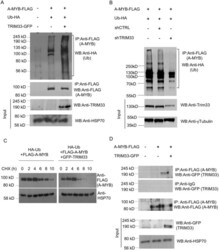
- Experimental details
- Fig 4 TRIM33 regulates A-MYB stability through ubiquitination. ( A ) HEK293T cells were transfected with HA-tagged ubiquitin and FLAG-tagged A-MYB in the presence or in the absence of GFP-tagged TRIM33. To detect ubiquitinated A-MYB, immunoprecipitation was performed on the lysates using anti-FLAG affinity beads and a Western blot was performed using anti-HA antibodies (top panel). To detect the immunoprecipitated A-MYB, the Western blot was probed with anti-FLAG antibodies (middle panel). Expression of tagged TRIM33 and the loading control HSP70 is shown in the bottom panel. ( B ) HEK293T cells were transfected with HA-tagged ubiquitin and FLAG-tagged A-MYB and either a control or TRIM33 shRNA expression vector. A-MYB was immunoprecipitated and Western blotting was performed as in ( A ) to detect ubiquitinated A-MYB (top panel). Lysates were subjected to Western blotting with anti-TRIM33 antibody to show knock down and anti-gamma-Tubulin antibody as a loading control (bottom panels). ( C ) The protein stability of A-MYB in the presence and in the absence of TRIM33 was monitored using the protein translation inhibitor cycloheximide (CHX). Cells were transfected as in ( A ) and CHX was added at 10 mug/ml and samples were analyzed by Western blots at various time points, as shown. HSP70 was used as the loading control. ( D ) FLAG-tagged A-MYB and GFP-tagged TRIM33 were co-expressed in HEK293T cells. Lysates were subjected to immunoprecipitation with anti-FLAG beads or, as a con
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
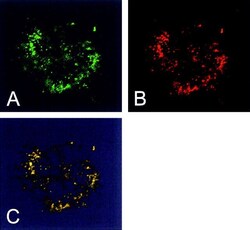
- Experimental details
- Figure 2 Bax staining appears in mitochondria. HeLa cells treated with 1 muM staurosporine for 2 h were double immunostained with antibodies against amino acids 11-30 of human Bax (Bax N-20 sc-493; Santa Cruz Biotechnology ) and mt-hsp-70. Fluorescence microscopy was performed using a LSM 410 confocal microscope ( Zeiss ). The two channels are shown separately (A, anti-Bax; B, anti-mt-hsp-70) and merged (C).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
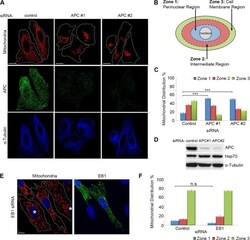
- Experimental details
- FIGURE 1: Loss of full-length APC induces perinuclear redistribution of mitochondria. (A) APC was silenced in U2OS cells by siRNA (APC #1 and #2), and mitochondrial distribution was analyzed by immunofluorescence microscopy after cells were stained for mitochondria (CMX-Ros) and APC. The microtubule network remained intact (alpha-tubulin). (B) The distribution of mitochondria in different ""zones"" was scored (C), revealing redistribution of mitochondria to the perinuclear region (zone 1) with APC siRNAs (***, p < 0.001). (D) Loss of APC in U2OS cells was confirmed by Western blot. (E) HDF1314 cells treated with EB1 siRNA were stained for mitochondrial distribution (CMX-Ros) and EB1. Cells displaying EB1 knockdown are indicated (*). (F) Scoring of mitochondrial distribution after EB1 silencing revealed no significant difference relative to control (n.s., not significant). Bar graph data are presented as mean (+-SD), statistical analysis by unpaired two-tailed t test with Bonferroni correction (C and F). Scale bars: 10 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
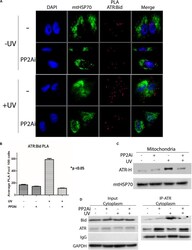
- Experimental details
- FIGURE 4 Inhibition of PP2A's dephosphorylation of pATR and ATR-L accumulation in the cytoplasm leads to reduced association of ATR with tBid on mitochondria. (A) PLA shows that cytoplasmic ATR directly associates with Bid and that this association increases with UV treatment, but the UV-induced association is attenuated by PP2A inhibitor treatment. The nuclei are stained with DAPI and mitochondria are indicated by mtHSP70 immunofluorescence. A549 cells were UV irradiated at 40 J/m 2 with a 2 h recovery. (B) A graphic display of the PLA data shown in (A) . The p < 0.05 refers to the sample (+UV, -PP2Ai) versus any of other samples. (C) ATR-H accumulation at the mitochondria increases with UV treatment at 40 J/m 2 in A549 cells, but is reduced in cells treated with PP2A inhibitor. (D) Biochemical confirmation of the reduction in the cytoplasmic ATR:tBid association observed by PLA (A,B) . A549 cells were pretreated with PP2Ai followed by UV irradiation with a 2 h recovery. After cellular fractionation ATR was immunoprecipitated from the cytoplasmic fraction using C-terminal-specific ATR antibody. The association of tBid with ATR was confirmed by WB.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
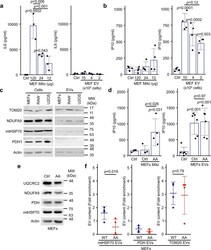
- Experimental details
- Fig. 1 Mitochondria and EVs activate distinct pro-inflammatory cytokines. a , b Extracellular mitochondria induce the secretion of pro-inflammatory cytokines. Mitochondria (Mito)(isolated from MEFs) or EVs (isolated by differential centrifugation from the media of MEFs grown for 24 h in EV-depleted media) were added to RAW cells in their culture media. The release of IL6 ( a ) and IP10 ( b ) into culture media was measured by ELISA 24 h. Individual points represent independent experiments ( a , n = 3; b , n = 4). Bars show the average +- SD. One-way ANOVA. c Representative western blot showing the amount of the specified mitochondrial proteins in the indicated cell types (20 ug) vs their EVs (5 ug). TOM20, outer membrane protein; NDUFA9, Complex I subunit; mtHSP70 matrix protein associated with the IM; PDH, matrix protein. Actin is used as a control, as it has been shown to associate with EVs. d AA-treated MEFs mitochondria stimulate IP10 production. RAW cells were treated as above with EVs (from 10 x 10 6 cells) and mitochondria (12 ug) isolated from Control or AA-treated MEFs, and IP10 release in culture media measured by ELISA. Individual points represent independent experiments (mitochondria, n = 4; EVs, n = 6). Bars show the average +- SD. One-way ANOVA. e AA treatment of WT MEFs causes the selective degradation of mitochondrial proteins. MEFs were treated with AA for 24 h and the indicated mitochondrial proteins analysed by western blot. f Enrichment of the indicated mi
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 6. HD-PTP and Rabaptin-5 interact via the ESCRT-III-binding site in HD-PTP and Rabaptin-5 CC2-2. (A) Schematic representation of key Rabaptin-5 and HD-PTP domains and interacting regions. The CHMP4/ESCRT-III-binding site in HD-PTP is highlighted by a dark circle. The minimal interacting region of Rabaptin-5 (RBPT5) identified by Y2H screen is underlined in bold. (B) Yeast cultures were co-transformed with full-length Rabaptin-5 and with the indicated HD-PTP constructs, and liquid cultures were streaked onto agar plates containing selection media as shown. DDO refers to double dropout medium, QDO refers to quadruple dropout medium. Representative data from three independent experiments. (C) Lysates from HEK293T cells co-transfected with GFP-Rabaptin-5 and HA-HD-PTP were immunoprecipitated (IP) with anti-HA and proteins detected by western blotting. Input is 2% of offered protein. Representative data from three independent experiments. (D) Lysates from HEK293T cells transfected with HA-HD-PTP were immunoprecipitated with anti-HA and proteins detected by western blotting. Input is 2% of offered protein. Representative data from three independent experiments. (E) Relocated HD-PTP recruits GFP-Rabaptin-5 to mitochondria. Top, schematic showing FKBP-HD-PTP-Myc relocating to mitochondria upon rapamycin-induced heterodimerisation of FKBP with the mitochondrially localised Mito-FRB. Bottom, immunofluorescence of HeLa cells expressing Mito-FRB, FKBP-HD-PTP-Myc and either GFP or GF
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
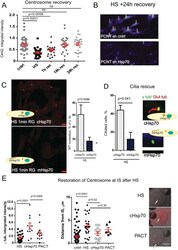
- Experimental details
- FIGURE 7: Hsp70 enables centrosome recovery, and protects centrosome functions after HS. (A) Semiquantitative analysis (x10 5 arbitrary units) of Centrin2 signal intensity shows centrosome disruption and recovery after HS during 24 h. (B) Semiquantitative intensity profiles of PCNT from cells recovered for 24 h at 37@C after HS demonstrate that Hsp70 depletion impairs centrosome recovery. (C) Confocal microscopy images demonstrating rescue of MT regrowth early after microtubule depolymerization (alpha-tubulin, 1 min of regrowth) after HS in cells expressing the centrosome targeting protein cHsp70 but not the membrane-targeting protein mHsp70. Percentage of the cells positive for detectable MT regrowth 1 min after HS exposure in RPE cell lines expressing cHsp70 or mHsp70 (five experiments, 400-500 cells/sample, mean +- SD). (D) Percentage of the cells with cilia after HS in cells expressing cHsp70 or mHsp70 (three experiments, 400-500 cells/sample, mean +- SD). Maximum projections of ciliated cells (cilia marker, glutamylated tubulin, red; centrosome marker, gamma-tubulin, green) after HS exposure in cells expressing cHsp70 or mHsp70. (E) Centrosomal Hsp70 protects centrosome from HS-induced damage in IS conjugates; left, x10 5 arbitrary units, 10-40 centrosomes/sample, mean +-S EM; middle, distance between centrosome and IS, micrometers. Right, gamma-tubulin, red, over DIC images; bar, 5 mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
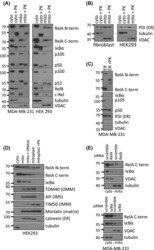
- Experimental details
- Figure 1 RelA and IkappaBalpha reside in mitochondria Protein levels from the indicated sub-cellular fractions were analysed by immunoblotting. Mitochondrial and cytosolic extracts were prepared from MDA-MB-231 ( A ) and HEK293 ( A, B ) cells by differential centrifugation. Both fractions were treated with 10 ng/ug of protein with proteinase K for 30 min on ice. ( C ) The ER from MDA-MB-231 cells was separated from crude mitochondrial extracts on a 15%/17.5%/20% OptiPrep gradient. Pure ER was collected in the 20% fraction and treated with 10 ng/ug of protein with proteinase K for 30 min on ice. ( D ) Mitochondria from HEK293 cells were treated with 0.2 U DNAse per mg of mitochondria for 15 min at room temperature and sub-fractionated by swelling with 10 mM Tris, pH 7.4. The remaining mitoplast containing only the inner mitochondrial membrane and the enclosed matrix was additionally treated with 5 ng/ug of protein with proteinase K from 30 min on ice. ( E ) RelA and I kappa B alpha were knocked down with siRNA in MDA-MB-231 cells and proteinase K-treated mitochondria were prepared as in A. Data are representative of a minimum of two independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
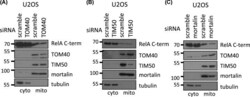
- Experimental details
- Figure 2 TOM40 and mortalin are required for the mitochondrial import of RelA Mitochondrial and cytosolic extracts were prepared by differential centrifugation from U2OS cells, where TOM40 ( A ), TIM50 ( B ) or mortalin ( C ) were knocked down with siRNA. The mitochondrial fractions were treated with 10 ng/ug of protein with proteinase K for 30 min on ice. Protein levels in each fraction were analysed by immunoblotting as indicated. Data are representative of a minimum of two independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
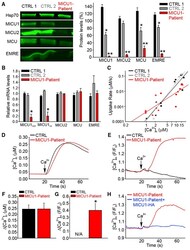
- Experimental details
- Figure 1. MCU Complex Composition and Mitochondrial Ca 2+ Handling in Fibroblasts from MICU1 Loss-of-Function Patients (A) Representative immunoblots of Hsp70, MICU1, MICU2, MCU, and EMRE in control (CTRL; CTRL1 and CTRL2) and MICU1-patient fibroblasts. Relative protein levels are displayed in the bar graph; each protein was normalized to Hsp70, and expressed relative to the mean of CTRL1 and CTRL2 fibroblasts (n = 3, *p < 0.05, **p < 0.01). (B) Relative mRNA levels of total MICU1 (MICU1 TOT ), transcript variant 3 of MICU1 (MICU1 v3 ), MICU2, MCU, and EMRE. Each transcript was normalized to beta-actin mRNA, and expressed relative to the mean of CTRL1 and CTRL2 fibroblasts (n = 3, *p < 0.05). Double-logarithmic plot of the initial rates of Ca 2+ uptake against the peak free [Ca 2+ ] c . Slope of each linear fit is indicated. The following total CaCl 2 additions were applied: 3, 4, 5, 6, 10, and 30 muM (mean +- SEM, n = 4). For calculation of the initial rates linear fits were used because exponentials could not be fit well when the uptake was small. However, the exponential fit is expected to be different from the linear fit when the Ca 2+ clearance is substantial, so we recalculated the uptake rates at [Ca 2+ ] c of 15 muM, where the CTRLs gave 0.116 +- 0.0176 muM/s and the MICU1-patient showed 0.026 +- 0.0038 muM/s (p < 0.05, Mann-Whitney), confirming a decrease in the mitochondrial Ca 2+ uptake in the MICU1-patient at > 10 muM [Ca 2+ ] c . (D and E) Mean time courses from
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
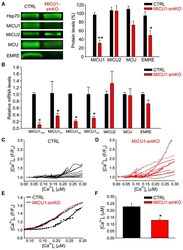
- Experimental details
- Figure 2. Ablation of MICU1 in Mouse SM (A) Representative immunoblots of Hsp70, MICU1, MICU2, MCU, and EMRE in SM from CTRL Micu1 F/F (CTRL, black bars) and muscle-specific (CK promoter-drive Cre recombinase) MICU1 knockout (MICU1-smKO, red bars) mice. Relative protein levels are displayed in the bar graph; each proteinwas normalized to Hsp70, and expressed relative to CTRL (mean +- SEM, n = 3, *p < 0.01, **p < 0.001). Relative mRNA levels of total MICU1 (MICU1tot), transcript variant 3 of MICU1 (MICU1 v3 ), transcript variant 2 of MICU1 (MICU1 v2 ; also known as MICU1.1), transcript variant 1 of MICU1 (MICU1 v1 ), MICU2, MCU, and EMRE in SM from CTRL and MICU1-smKO mice. Each transcript was normalized to beta-actin mRNA and expressed relative to CTRL (mean +- SEM, n = 3, *p < 0.05, Student's t test). (C and D) [Ca 2+ ] m (mtRCaMP) versus [Ca 2+ ] c (fura2) curves from individual CTRL (C) and MICU1-smKO (D) isolated SM fibers. Fibers were pretreated with Tg in a Ca 2+ -free ECM. To evoke SOCE, 2 mM CaCl 2 (Ca 2+ ) was added (n = 15-16 fibers). (E) Mean traces of the [Ca 2+ ] m responses versus SOCE-associated [Ca (2) c for CTRL and MICU1-smKO from (D) and (E), respectively. [Ca 2+ ] c at which CTRL and MICU1-smKO fibers showed a 10% increase in [Ca 2+ ] m (expressed as F/F 0 of mtRCaMP). Experiments were as in (C) and (D).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry