Antibody data
- Antibody Data
- Antigen structure
- References [42]
- Comments [0]
- Validations
- Immunocytochemistry [8]
- Immunoprecipitation [1]
- Immunohistochemistry [3]
- Flow cytometry [6]
- Other assay [15]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-414 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- p23 Monoclonal Antibody (JJ3)
- Antibody type
- Monoclonal
- Antigen
- Recombinant full-length protein
- Description
- MA3-414 detects p23 from chicken, guinea pig, human, rat, mouse and rabbit samples. MA3-414 has been successfully used in Western blot, immunofluorescence and immunoprecipitation procedures. By Western blot, this antibody detects a 19 or 23 kDa protein representing p23 from rabbit reticulocyte lysate. MA3-414 can be used to immunoprecipitate both free and complexed p23. The MA3-414 antigen is recombinant human p23.
- Reactivity
- Human, Mouse, Rat, Chicken/Avian, Guinea Pig, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- JJ3
- Vial size
- 100 μL
- Concentration
- Conc. Not Determined
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references The Alpha Isoform of Heat Shock Protein 90 and the Co-chaperones p23 and Cdc37 Promote Opioid Anti-nociception in the Brain.
In silico identification and biochemical characterization of the human dicarboxylate clamp TPR protein interaction network.
Hsp70 cochaperones HspBP1 and BAG-1M differentially regulate steroid hormone receptor function.
Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis.
pRb controls estrogen receptor alpha protein stability and activity.
Hsp90 cochaperones p23 and FKBP4 physically interact with hAgo2 and activate RNA interference-mediated silencing in mammalian cells.
p23 co-chaperone protects the aryl hydrocarbon receptor from degradation in mouse and human cell lines.
Type 1 inositol-1,4,5-trisphosphate receptor is a late substrate of caspases during apoptosis.
Caspase-1 promiscuity is counterbalanced by rapid inactivation of processed enzyme.
Molecular determinants involved in activation of caspase 7.
ATP binding to Hsp90 is sufficient for effective chaperoning of p53 protein.
Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth.
High levels of Hsp90 cochaperone p23 promote tumor progression and poor prognosis in breast cancer by increasing lymph node metastases and drug resistance.
The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events.
Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors.
Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells.
Glucose deprivation induces an atypical form of apoptosis mediated by caspase-8 in Bax-, Bak-deficient cells.
Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta.
Expression of Hsp90 chaperone [corrected] proteins in human tumor tissue.
17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse.
Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway.
A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates.
Multiple 40-kDa heat-shock protein chaperones function in Tom70-dependent mitochondrial import.
Hsp90 regulates the Fanconi anemia DNA damage response pathway.
The cochaperone p23 differentially regulates estrogen receptor target genes and promotes tumor cell adhesion and invasion.
Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease.
Redefining the role of the endogenous XAP2 and C-terminal hsp70-interacting protein on the endogenous Ah receptors expressed in mouse and rat cell lines.
17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration.
Expression and localization of adipophilin and perilipin in human fetal membranes: association with lipid bodies and enzymes involved in prostaglandin synthesis.
FK506 requires stimulation of the extracellular signal-regulated kinase 1/2 and the steroid receptor chaperone protein p23 for neurite elongation.
Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1.
Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes.
Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes.
Progesterone receptor deficient in chromatin binding has an altered cellular state.
GERp95 belongs to a family of signal-transducing proteins and requires Hsp90 activity for stability and Golgi localization.
The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties.
The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties.
Active participation of Hsp90 in the biogenesis of the trimeric reovirus cell attachment protein sigma1.
Active participation of Hsp90 in the biogenesis of the trimeric reovirus cell attachment protein sigma1.
Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids.
A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23.
Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes.
Lei W, Duron DI, Stine C, Mishra S, Blagg BSJ, Streicher JM
Frontiers in molecular neuroscience 2019;12:294
Frontiers in molecular neuroscience 2019;12:294
In silico identification and biochemical characterization of the human dicarboxylate clamp TPR protein interaction network.
Bernadotte A, Kumar R, Winblad B, Pavlov PF
FEBS open bio 2018 Nov;8(11):1830-1843
FEBS open bio 2018 Nov;8(11):1830-1843
Hsp70 cochaperones HspBP1 and BAG-1M differentially regulate steroid hormone receptor function.
Knapp RT, Wong MJ, Kollmannsberger LK, Gassen NC, Kretzschmar A, Zschocke J, Hafner K, Young JC, Rein T
PloS one 2014;9(1):e85415
PloS one 2014;9(1):e85415
Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis.
Donnelly BF, Needham PG, Snyder AC, Roy A, Khadem S, Brodsky JL, Subramanya AR
The Journal of biological chemistry 2013 May 3;288(18):13124-35
The Journal of biological chemistry 2013 May 3;288(18):13124-35
pRb controls estrogen receptor alpha protein stability and activity.
Caligiuri I, Toffoli G, Giordano A, Rizzolio F
Oncotarget 2013 Jun;4(6):875-83
Oncotarget 2013 Jun;4(6):875-83
Hsp90 cochaperones p23 and FKBP4 physically interact with hAgo2 and activate RNA interference-mediated silencing in mammalian cells.
Pare JM, LaPointe P, Hobman TC
Molecular biology of the cell 2013 Aug;24(15):2303-10
Molecular biology of the cell 2013 Aug;24(15):2303-10
p23 co-chaperone protects the aryl hydrocarbon receptor from degradation in mouse and human cell lines.
Nguyen PM, Wang D, Wang Y, Li Y, Uchizono JA, Chan WK
Biochemical pharmacology 2012 Sep 15;84(6):838-50
Biochemical pharmacology 2012 Sep 15;84(6):838-50
Type 1 inositol-1,4,5-trisphosphate receptor is a late substrate of caspases during apoptosis.
Elkoreh G, Blais V, Béliveau E, Guillemette G, Denault JB
Journal of cellular biochemistry 2012 Aug;113(8):2775-84
Journal of cellular biochemistry 2012 Aug;113(8):2775-84
Caspase-1 promiscuity is counterbalanced by rapid inactivation of processed enzyme.
Walsh JG, Logue SE, Lüthi AU, Martin SJ
The Journal of biological chemistry 2011 Sep 16;286(37):32513-24
The Journal of biological chemistry 2011 Sep 16;286(37):32513-24
Molecular determinants involved in activation of caspase 7.
Boucher D, Blais V, Drag M, Denault JB
Bioscience reports 2011 Aug;31(4):283-94
Bioscience reports 2011 Aug;31(4):283-94
ATP binding to Hsp90 is sufficient for effective chaperoning of p53 protein.
Walerych D, Gutkowska M, Klejman MP, Wawrzynow B, Tracz Z, Wiech M, Zylicz M, Zylicz A
The Journal of biological chemistry 2010 Oct 15;285(42):32020-8
The Journal of biological chemistry 2010 Oct 15;285(42):32020-8
Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth.
Quintá HR, Maschi D, Gomez-Sanchez C, Piwien-Pilipuk G, Galigniana MD
Journal of neurochemistry 2010 Nov;115(3):716-34
Journal of neurochemistry 2010 Nov;115(3):716-34
High levels of Hsp90 cochaperone p23 promote tumor progression and poor prognosis in breast cancer by increasing lymph node metastases and drug resistance.
Simpson NE, Lambert WM, Watkins R, Giashuddin S, Huang SJ, Oxelmark E, Arju R, Hochman T, Goldberg JD, Schneider RJ, Reiz LF, Soares FA, Logan SK, Garabedian MJ
Cancer research 2010 Nov 1;70(21):8446-56
Cancer research 2010 Nov 1;70(21):8446-56
The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events.
Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G
Molecular and cellular biology 2010 Mar;30(5):1285-98
Molecular and cellular biology 2010 Mar;30(5):1285-98
Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors.
Schülke JP, Wochnik GM, Lang-Rollin I, Gassen NC, Knapp RT, Berning B, Yassouridis A, Rein T
PloS one 2010 Jul 22;5(7):e11717
PloS one 2010 Jul 22;5(7):e11717
Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells.
Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF
Apoptosis : an international journal on programmed cell death 2010 Dec;15(12):1480-93
Apoptosis : an international journal on programmed cell death 2010 Dec;15(12):1480-93
Glucose deprivation induces an atypical form of apoptosis mediated by caspase-8 in Bax-, Bak-deficient cells.
Caro-Maldonado A, Tait SW, Ramírez-Peinado S, Ricci JE, Fabregat I, Green DR, Muñoz-Pinedo C
Cell death and differentiation 2010 Aug;17(8):1335-44
Cell death and differentiation 2010 Aug;17(8):1335-44
Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta.
Echeverría PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien Pilipuk G, Galigniana MD
Molecular and cellular biology 2009 Sep;29(17):4788-97
Molecular and cellular biology 2009 Sep;29(17):4788-97
Expression of Hsp90 chaperone [corrected] proteins in human tumor tissue.
McDowell CL, Bryan Sutton R, Obermann WM
International journal of biological macromolecules 2009 Oct 1;45(3):310-4
International journal of biological macromolecules 2009 Oct 1;45(3):310-4
17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse.
Tokui K, Adachi H, Waza M, Katsuno M, Minamiyama M, Doi H, Tanaka K, Hamazaki J, Murata S, Tanaka F, Sobue G
Human molecular genetics 2009 Mar 1;18(5):898-910
Human molecular genetics 2009 Mar 1;18(5):898-910
Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway.
Inoue S, Browne G, Melino G, Cohen GM
Cell death and differentiation 2009 Jul;16(7):1053-61
Cell death and differentiation 2009 Jul;16(7):1053-61
A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates.
Taherian A, Krone PH, Ovsenek N
Biochemistry and cell biology = Biochimie et biologie cellulaire 2008 Feb;86(1):37-45
Biochemistry and cell biology = Biochimie et biologie cellulaire 2008 Feb;86(1):37-45
Multiple 40-kDa heat-shock protein chaperones function in Tom70-dependent mitochondrial import.
Bhangoo MK, Tzankov S, Fan AC, Dejgaard K, Thomas DY, Young JC
Molecular biology of the cell 2007 Sep;18(9):3414-28
Molecular biology of the cell 2007 Sep;18(9):3414-28
Hsp90 regulates the Fanconi anemia DNA damage response pathway.
Oda T, Hayano T, Miyaso H, Takahashi N, Yamashita T
Blood 2007 Jun 1;109(11):5016-26
Blood 2007 Jun 1;109(11):5016-26
The cochaperone p23 differentially regulates estrogen receptor target genes and promotes tumor cell adhesion and invasion.
Oxelmark E, Roth JM, Brooks PC, Braunstein SE, Schneider RJ, Garabedian MJ
Molecular and cellular biology 2006 Jul;26(14):5205-13
Molecular and cellular biology 2006 Jul;26(14):5205-13
Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease.
Maida ME, Hurley SD, Daeschner JA, Moore AH, O'Banion MK
Brain research 2006 Aug 4;1103(1):164-72
Brain research 2006 Aug 4;1103(1):164-72
Redefining the role of the endogenous XAP2 and C-terminal hsp70-interacting protein on the endogenous Ah receptors expressed in mouse and rat cell lines.
Pollenz RS, Dougherty EJ
The Journal of biological chemistry 2005 Sep 30;280(39):33346-56
The Journal of biological chemistry 2005 Sep 30;280(39):33346-56
17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration.
Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G
Nature medicine 2005 Oct;11(10):1088-95
Nature medicine 2005 Oct;11(10):1088-95
Expression and localization of adipophilin and perilipin in human fetal membranes: association with lipid bodies and enzymes involved in prostaglandin synthesis.
Meadows JW, Pitzer B, Brockman DE, Myatt L
The Journal of clinical endocrinology and metabolism 2005 Apr;90(4):2344-50
The Journal of clinical endocrinology and metabolism 2005 Apr;90(4):2344-50
FK506 requires stimulation of the extracellular signal-regulated kinase 1/2 and the steroid receptor chaperone protein p23 for neurite elongation.
Gold BG, Zhong YP
Neuro-Signals 2004 May-Jun;13(3):122-9
Neuro-Signals 2004 May-Jun;13(3):122-9
Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1.
Subbaramaiah K, Yoshimatsu K, Scherl E, Das KM, Glazier KD, Golijanin D, Soslow RA, Tanabe T, Naraba H, Dannenberg AJ
The Journal of biological chemistry 2004 Mar 26;279(13):12647-58
The Journal of biological chemistry 2004 Mar 26;279(13):12647-58
Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes.
Stavreva DA, Müller WG, Hager GL, Smith CL, McNally JG
Molecular and cellular biology 2004 Apr;24(7):2682-97
Molecular and cellular biology 2004 Apr;24(7):2682-97
Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes.
Stavreva DA, Müller WG, Hager GL, Smith CL, McNally JG
Molecular and cellular biology 2004 Apr;24(7):2682-97
Molecular and cellular biology 2004 Apr;24(7):2682-97
Progesterone receptor deficient in chromatin binding has an altered cellular state.
Botos J, Xian W, Smith DF, Smith CL
The Journal of biological chemistry 2004 Apr 9;279(15):15231-9
The Journal of biological chemistry 2004 Apr 9;279(15):15231-9
GERp95 belongs to a family of signal-transducing proteins and requires Hsp90 activity for stability and Golgi localization.
Tahbaz N, Carmichael JB, Hobman TC
The Journal of biological chemistry 2001 Nov 16;276(46):43294-9
The Journal of biological chemistry 2001 Nov 16;276(46):43294-9
The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties.
Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO
The Journal of biological chemistry 2000 Feb 4;275(5):3305-12
The Journal of biological chemistry 2000 Feb 4;275(5):3305-12
The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties.
Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO
The Journal of biological chemistry 2000 Feb 4;275(5):3305-12
The Journal of biological chemistry 2000 Feb 4;275(5):3305-12
Active participation of Hsp90 in the biogenesis of the trimeric reovirus cell attachment protein sigma1.
Gilmore R, Coffey MC, Lee PW
The Journal of biological chemistry 1998 Jun 12;273(24):15227-33
The Journal of biological chemistry 1998 Jun 12;273(24):15227-33
Active participation of Hsp90 in the biogenesis of the trimeric reovirus cell attachment protein sigma1.
Gilmore R, Coffey MC, Lee PW
The Journal of biological chemistry 1998 Jun 12;273(24):15227-33
The Journal of biological chemistry 1998 Jun 12;273(24):15227-33
Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids.
Hu J, Toft DO, Seeger C
The EMBO journal 1997 Jan 2;16(1):59-68
The EMBO journal 1997 Jan 2;16(1):59-68
A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23.
Johnson JL, Toft DO
The Journal of biological chemistry 1994 Oct 7;269(40):24989-93
The Journal of biological chemistry 1994 Oct 7;269(40):24989-93
Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes.
Johnson JL, Beito TG, Krco CJ, Toft DO
Molecular and cellular biology 1994 Mar;14(3):1956-63
Molecular and cellular biology 1994 Mar;14(3):1956-63
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
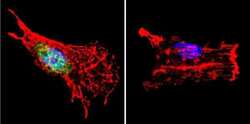
- Experimental details
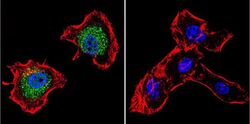
- Immunofluorescent analysis of p23 using Anti-p23 Monoclonal Antibody (JJ5) (Product # MA3-414) shows staining in C6 Cells. p23 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing p23 (Product # MA3-414) at a dilution of 1:500 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
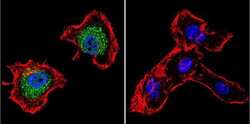
- Experimental details
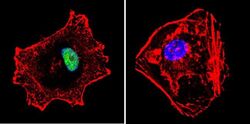
- Immunofluorescent analysis of p23 using Anti-p23 Monoclonal Antibody (JJ5) (Product # MA3-414) shows staining in Hela Cells. p23 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing p23 (Product # MA3-414) at a dilution of 1:500 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
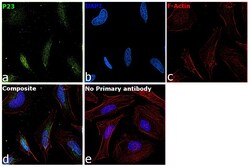
- Experimental details
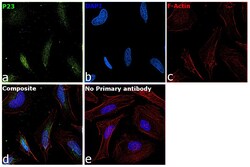
- Immunofluorescence analysis of p23 was performed using 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with p23 Monoclonal Antibody (JJ3) (Product # MA3-414) at 1:200 in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647 (Product # A32787), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Blue) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Nucleus and cytoplasmic localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60x magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
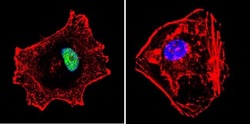
- Experimental details
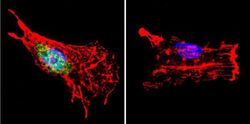
- Immunofluorescent analysis of p23 using Anti-p23 Monoclonal Antibody (JJ5) (Product # MA3-414) shows staining in MCF-7 Cells. p23 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing p23 (Product # MA3-414) at a dilution of 1:500 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of p23 using Anti-p23 Monoclonal Antibody (JJ5) (Product # MA3-414) shows staining in Hela Cells. p23 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing p23 (Product # MA3-414) at a dilution of 1:500 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of p23 using Anti-p23 Monoclonal Antibody (JJ5) (Product # MA3-414) shows staining in MCF-7 Cells. p23 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing p23 (Product # MA3-414) at a dilution of 1:500 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of p23 using Anti-p23 Monoclonal Antibody (JJ5) (Product # MA3-414) shows staining in C6 Cells. p23 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing p23 (Product # MA3-414) at a dilution of 1:500 over night at 4°C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescence analysis of p23 was performed using 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with p23 Monoclonal Antibody (JJ3) (Product # MA3-414) at 1:200 in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647 (Product # A32787), (1:2000 dilution), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Blue) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing Nucleus and cytoplasmic localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60x magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
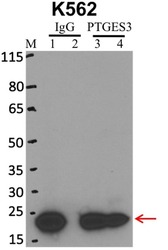
- Immunoprecipitation of p23 was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of p23 monoclonal antibody (Product # MA3-414) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a p23 monoclonal antibody (Product # MA3-414) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
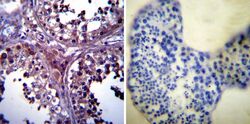
- Immunohistochemistry was performed on normal biopsies of deparaffinized Human testis tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:1000 with a mouse monoclonal antibody recognizing p23 (Product # MA3-414) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
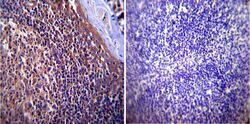
- Immunohistochemistry was performed on normal biopsies of deparaffinized Human tonsil tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:1000 with a mouse monoclonal antibody recognizing p23 (Product # MA3-414) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
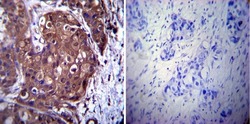
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human breast carcinoma tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:1000 with a mouse monoclonal antibody recognizing p23 (Product # MA3-414) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
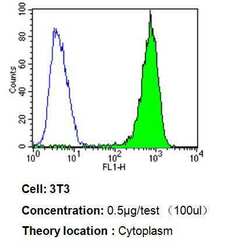
- Experimental details
- Flow cytometry analysis of p23 in 3T3 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with p23 monoclonal antibody (Product # MA3-414) at a dilution of 0.5 µg/test for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
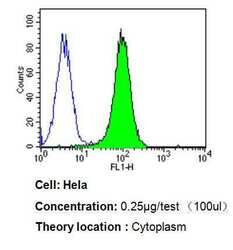
- Experimental details
- Flow cytometry analysis of p23 in Hela cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with p23 monoclonal antibody (Product # MA3-414) at a dilution of 0.25 µg/test for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
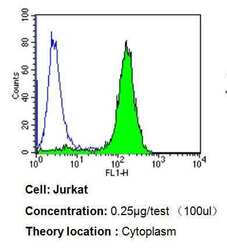
- Experimental details
- Flow cytometry analysis of p23 in Jurkat cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with p23 monoclonal antibody (Product # MA3-414) at a dilution of 0.25 µg/test for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
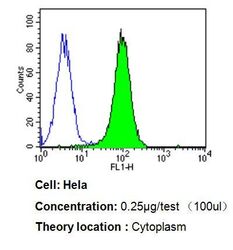
- Experimental details
- Flow cytometry analysis of p23 in Hela cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with p23 monoclonal antibody (Product # MA3-414) at a dilution of 0.25 µg/test for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
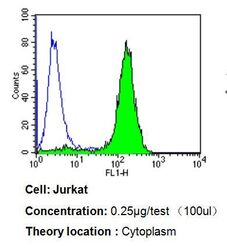
- Experimental details
- Flow cytometry analysis of p23 in Jurkat cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with p23 monoclonal antibody (Product # MA3-414) at a dilution of 0.25 µg/test for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
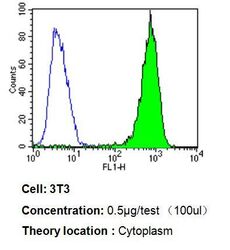
- Experimental details
- Flow cytometry analysis of p23 in 3T3 cells compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde, washed with PBS, and incubated with p23 monoclonal antibody (Product # MA3-414) at a dilution of 0.5 µg/test for 60 min at room temperature. Cells were then blocked in a solution of 2% BSA-PBS for 30 min at room temperature, incubated for 40 min at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody, and re-suspended in PBS for FACS analysis.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
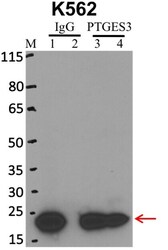
- Experimental details
- RNA immunoprecipitation (RIP) western of p23 was performed on K562 cells. Antigen-antibody complexes were formed by incubating approximately 500 µg whole cell lysate with 5 µg of p23 monoclonal antibody (Product # MA3-414) rotating 60 min at RT. The immune complexes were captured on 625 µg of anti-mouse coated Dynabeads (Product # 11202D), washed extensively, and eluted with NuPAGE™ LDS Sample Buffer (Product # NP0007). Samples were resolved onto NuPAGE™ 4-12% Bis-Tris gel (Product # NP0335BOX). Lanes 1 and 3 are input and lanes 2 and 4 are IP. Proteins were transferred to PVDF membrane (Product # IB23001). Membrane was blocked in 5% milk. Target was detected using a p23 monoclonal antibody (Product # MA3-414) at a dilution of 1:2000, followed by a 1:4000 dilution of secondary antibody. Chemiluminescent detection was performed using ECL Western Blotting Substrate (Product # 32106). Data courtesy of the Yeo lab as part of the ENCODE project (www.encodeproject.org).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
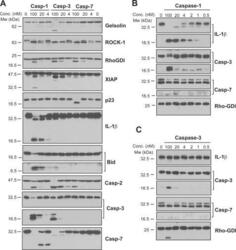
- Experimental details
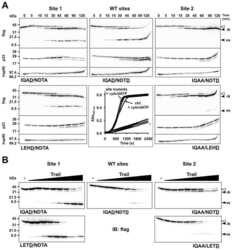
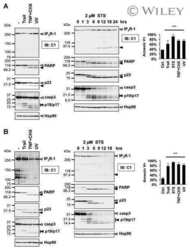
- FIGURE 4. Caspase-1 exhibits greater promiscuity toward natural substrates than caspases-3 or -7. A , processing of substrate proteins in Jurkat cell-free extracts after addition of the indicated amounts of recombinant caspase ( Casp )-1, -3, and -7 followed by incubation for 2 h at 37 degC. Recombinant caspases were active site titrated (see supplemental Fig. S1 ) as described under ""Experimental Procedures."" Recombinant IL-1beta (10 ng per reaction) was added to cell-free extracts as a positive control for caspase-1 activity. Substrate proteolysis was detected by SDS-PAGE followed by immunoblotting. B , caspase-1-mediated processing of the purified recombinant proteins over the indicated concentration ( Conc. ) range. The indicated proteins were incubated with recombinant active caspase-1 for 2 h at 37 degC. C , caspase-3-mediated processing of the purified recombinant proteins over the indicated concentration range. The indicated proteins were incubated with recombinant active caspase-3 for 2 h at 37 degC. Results are representative of at least three independent experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
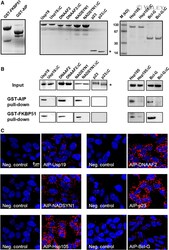
- Experimental details
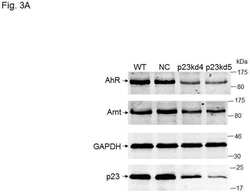
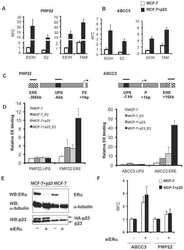
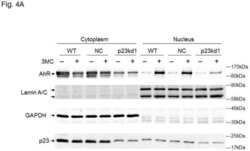
- In vitro and in situ interactions of Usp19, DNAAF 2, NADSYN 1, p23/ PTGES 3, Hsp105 and Bcl-G with dc TPR proteins. (A) Coomassie blue R staining of overexpressed and purified proteins used in GST pull-down assay. (B) Western blot analysis of GST- AIP and GST- FKBP 51 pull-down of Usp19, DNAAF 2, NADSYN 1, p23/ PTGES 3, Hsp105, Bcl-G and their truncated versions lacking five utmost C-terminal residues. (C) PLA of Usp19, DNAAF 2, NADSYN 1, p23/ PTGES 3, Hsp105 and Bcl-G interacting with AIP in HEK 293 cells. The asterisk indicates position of truncated p23 on the gel.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
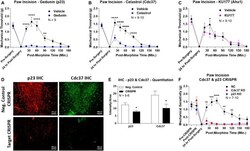
- Experimental details
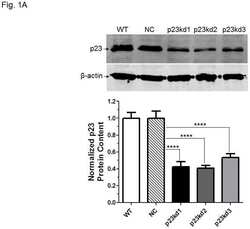
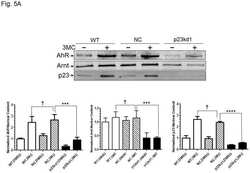
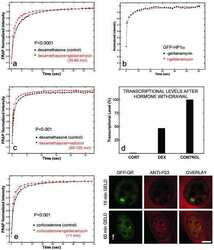
- Figure 4 Identification of the co-chaperones p23 and Cdc37 as promoters of opioid anti-nociception in the brain. Male CD-1 mice used for all experiments, data reported as the mean +- SEM, with sample sizes of mice/group noted in each graph. (A-C) Ten nanomoles of Gedunin (p23, A ), 10 nmol of Celastrol (Cdc37, B ), or 0.1 nmol of KU177 (Aha1, C ) or Vehicle control injected icv concurrently with paw incision surgery with a 24 h recovery, followed by 3.2 mg/kg morphine sc . Experiments performed with two technical replicates for each drug. *,**,**** p < 0.05, 0.01, 0.0001 vs. same time point inhibitor treatment group by two-way ANOVA with Fisher's Least Significant Difference post hoc test. (D) p23, Cdc37, or Negative Control CRISPR-treated mice with icv delivery of constructs analyzed by IHC for target knockdown on day 10. Representative images shown from the PRN. Both targets (p23 - red, Cdc37 - green) have a similar staining pattern to Hsp90alpha, and both are clearly reduced by CRISPR treatment. (E) Data from (D) for all mice quantitated as described in the ""Materials and Methods"" section. All mice treated in one technical replicate, with staining and analysis of the resulting tissue performed in more than one technical replicate. * p < 0.05 vs. same target Negative Control group by Unpaired 2-Tailed t -test. CRISPR treatment reduced p23 signal by 36.3% and Cdc37 by 46.0%. (F) CRISPR-treated mice had paw incision surgery performed on day 9, with injection of 3.2 mg/kg mo
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
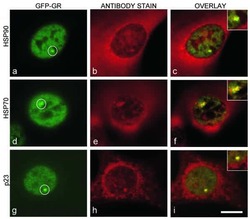
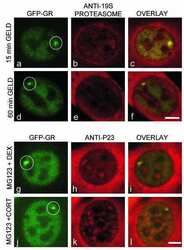
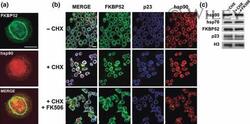
- 4 The annular ring of chaperones is a pre-assembled structure. (a) Undifferentiated N2a cells were stained with the Ac88 clone anti-hsp90 (red), which does not recognize the chaperone unless it is free or denatured. FKBP52 was used as marker of the perinuclear ring (green). (b) Indirect immunofluorescence for N2a cells pre-incubated for 30 min with cycloheximide (+CHX) followed by stimulation with FK506 for 3 h (+CHX + FK506) without changing the medium. (-CHX): Control of untreated cells. Bars = 10 mum.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry