Antibody data
- Antibody Data
- Antigen structure
- References [1]
- Comments [0]
- Validations
- Immunocytochemistry [2]
- Immunohistochemistry [1]
- Other assay [1]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA5-20333 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- CRTH2 Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- A suggested positive control is human heart tissue lysate. PA5-20333 can be used with blocking peptide PEP-0453.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- Maintain refrigerated at 2-8°C for up to 3 months. For long term storage store at -20°C
Submitted references AMPK induces regulatory innate lymphoid cells after traumatic brain injury.
Baban B, Braun M, Khodadadi H, Ward A, Alverson K, Malik A, Nguyen K, Nazarian S, Hess DC, Forseen S, Post AF, Vale FL, Vender JR, Hoda MN, Akbari O, Vaibhav K, Dhandapani KM
JCI insight 2021 Jan 11;6(1)
JCI insight 2021 Jan 11;6(1)
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
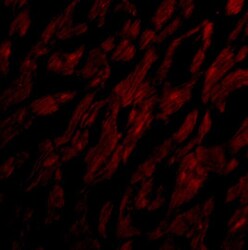
- Experimental details
- Immunofluorescence of CRTH2 in Human Heart cells with CRTH2 Polyclonal Antibody (Product # PA5-20333) at 20 µg/mL.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
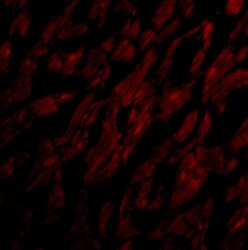
- Experimental details
- Immunofluorescence of CRTH2 in Human Heart cells with CRTH2 Polyclonal Antibody (Product # PA5-20333) at 20 µg/mL.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
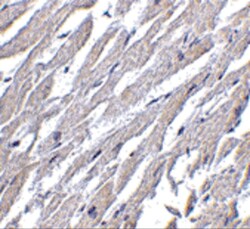
- Experimental details
- Immunohistochemistry of CRTH2 in human heart tissue with CRTH2 Polyclonal Antibody (Product # PA5-20333) at 2.5 µg/mL.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
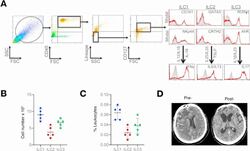
- Figure 1 Presence and frequency of ILC subtypes within the meninges of severe TBI patients. ( A ) Dura was collected from consecutive, severe TBI patients undergoing decompressive craniectomy to alleviate elevated intracranial pressure. ILCs were sorted using forward scatter (FSC)/side scatter (SSC) and identified as CD45 + , lineage-negative (Lin - ), CD127 + lymphoid cells. ILCs subtypes were further defined as ILC1:, CD45 + Lin - CD127 + CD161 + NKp44 + ; ILC2, CD45 + Lin - CD127 + GATA3 + CRTH2 + ; and ILC3, CD45 + Lin - CD127 + RORgammat + AhR + , as shown in representative flow cytometry scatterplots. Gray shaded areas indicate isotype controls. To demonstrate functionality, ILCs were further stimulated with cytokine cocktails, and production of signature cytokines was assessed (ILC1, IFN-gamma; ILC2, IL-5/IL-13; ILC3, IL-17). ( B and C ) Frequency of ILC subtypes from individual patients, expressed as total cell number ( B ) and % leukocytes ( C ) ( n = 5). Scatterplots depict mean +- SD. ( D ) Computed tomography scan of a TBI patient before (Pre-) and after (Post-) decompressive craniectomy surgery. The dura was collected during surgery at the time of bone flap removal.
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry