Antibody data
- Antibody Data
- Antigen structure
- References [99]
- Comments [0]
- Validations
- Immunocytochemistry [8]
- Immunohistochemistry [3]
- Other assay [42]
Submit
Validation data
Reference
Comment
Report error
- Product number
- MA3-915 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- ATP1A3 Monoclonal Antibody (XVIF9-G10)
- Antibody type
- Monoclonal
- Antigen
- Other
- Description
- MA3-915 detects sodium/potassium ATPase from human, monkey, bovine, sheep, canine, rabbit, guinea pig, mouse and rat tissue. This antibody is specific for the alpha-3 subunit. MA3-915 has been successfully used in Western blot and immunohistochemical procedures. By Western blot, this antibody detects an ~110 kDa protein representing the alpha-3 subunit of the sodium/potassium ATPase from canine skeletal muscle extract. Immunohistochemical staining of sodium/potassium ATPase in rat retina with MA3-915 yields a pattern consistent with plasma membrane localization. The MA3-915 antigen is canine cardiac microsomes.
- Reactivity
- Human, Mouse, Rat, Bovine, Canine, Guinea Pig, Rabbit
- Host
- Mouse
- Isotype
- IgG
- Antibody clone number
- XVIF9-G10
- Vial size
- 100 μg
- Concentration
- 1 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references The cellular prion protein interacts with and promotes the activity of Na,K-ATPases.
Disposition of Proteins and Lipids in Synaptic Membrane Compartments Is Altered in Q175/Q7 Huntington's Disease Mouse Striatum.
An interaction between PRRT2 and Na(+)/K(+) ATPase contributes to the control of neuronal excitability.
Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea.
Differential Membrane Binding and Seeding of Distinct α-Synuclein Fibrillar Polymorphs.
Effects of testosterone suppression, hindlimb immobilization, and recovery on [(3)H]ouabain binding site content and Na(+), K(+)-ATPase isoforms in rat soleus muscle.
Astrocyte deletion of α2-Na/K ATPase triggers episodic motor paralysis in mice via a metabolic pathway.
Resistance training upregulates skeletal muscle Na(+), K(+)-ATPase content, with elevations in both α(1) and α(2), but not β isoforms.
Identification of the retinoschisin-binding site on the retinal Na/K-ATPase.
Characterization of transgenic mouse lines for labeling type I and type II afferent neurons in the cochlea.
Proteomic Analysis of Dendritic Filopodia-Rich Fraction Isolated by Telencephalin and Vitronectin Interaction.
Differential Expression of Ca(2+)-buffering Protein Calretinin in Cochlear Afferent Fibers: A Possible Link to Vulnerability to Traumatic Noise.
Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins.
Tyrosine Hydroxylase Expression in Type II Cochlear Afferents in Mice.
Gestation changes sodium pump isoform expression, leading to changes in ouabain sensitivity, contractility, and intracellular calcium in rat uterus.
Retinoschisin is linked to retinal Na/K-ATPase signaling and localization.
Sodium pump organization in dendritic spines.
Dissociation between short-term unloading and resistance training effects on skeletal muscle Na+,K+-ATPase, muscle function, and fatigue in humans.
Cell specific differences in the protein abundances of GAPDH and Na(+),K(+)-ATPase in skeletal muscle from aged individuals.
Characterization of Fatty Acid Binding Protein 7 (FABP7) in the Murine Retina.
Ouabain-Induced Signaling and Cell Survival in SK-N-SH Neuroblastoma Cells Differentiated by Retinoic Acid.
Single-fiber expression and fiber-specific adaptability to short-term intense exercise training of Na+-K+-ATPase α- and β-isoforms in human skeletal muscle.
The effects of knee injury on skeletal muscle function, Na+, K+-ATPase content, and isoform abundance.
Functional Interaction Between Na/K-ATPase and NMDA Receptor in Cerebellar Neurons.
Identification of a VxP Targeting Signal in the Flagellar Na+ /K+ -ATPase.
Regional ion channel gene expression heterogeneity and ventricular fibrillation dynamics in human hearts.
Alternative splice isoforms of small conductance calcium-activated SK2 channels differ in molecular interactions and surface levels.
Distribution of Na,K-ATPase α subunits in rat vestibular sensory epithelia.
Numb regulates the polarized delivery of cyclic nucleotide-gated ion channels in rod photoreceptor cilia.
Agonist-dependent and -independent dopamine-1-like receptor signalling differentially regulates downstream effectors.
Regulation and pharmacological blockade of sodium-potassium ATPase: a novel pathway to neuropathy.
The effects of osteoarthritis and age on skeletal muscle strength, Na+-K+-ATPase content, gene and isoform expression.
Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons.
Overexpression of the polycystin-1 C-tail enhances sensitivity of M-1 cells to ouabain.
A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3.
Cardiac glycosides block cancer growth through HIF-1α- and NF-κB-mediated Plk1.
Unchanged [3H]ouabain binding site content but reduced Na+-K+ pump α2-protein abundance in skeletal muscle in older adults.
Impaired exercise performance and muscle Na(+),K(+)-pump activity in renal transplantation and haemodialysis patients.
Nearest neighbor analysis of dopamine D1 receptors and Na(+)-K(+)-ATPases in dendritic spines dissected by STED microscopy.
mGluR6 deletion renders the TRPM1 channel in retina inactive.
Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor.
In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models.
Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2.
Spatial distribution of Na+-K+-ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy.
Aldosterone increases Na+ -K+ -ATPase activity in skeletal muscle of patients with Conn's syndrome.
Changes in Na+, K+-ATPase activity and alpha 3 subunit expression in CNS after administration of Na+, K+-ATPase inhibitors.
Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts.
BK channels mediate cholinergic inhibition of high frequency cochlear hair cells.
Immunostaining for the α3 isoform of the Na+/K+-ATPase is selective for functionally identified muscle spindle afferents in vivo.
Increase in ouabain-sensitive K+-ATPase activity in hepatocellular carcinoma by overexpression of Na+, K+-ATPase alpha 3-isoform.
Mutant huntingtin and glycogen synthase kinase 3-beta accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington's disease.
The ouabain-binding site of the α2 isoform of Na,K-ATPase plays a role in blood pressure regulation during pregnancy.
Enhanced pressor response to increased CSF sodium concentration and to central ANG I in heterozygous alpha2 Na+ -K+ -ATPase knockout mice.
Distribution of the Na,K-ATPase alpha subunit in the rat spiral ganglion and organ of corti.
Glutamate transporter coupling to Na,K-ATPase.
Ca2+ influx mechanisms in caveolae vesicles of pulmonary smooth muscle plasma membrane under inhibition of alpha2beta1 isozyme of Na+/K+-ATPase by ouabain.
Over-expression of translationally controlled tumor protein in lens epithelial cells seems to be associated with cataract development.
Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries.
Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex.
Muscle Na+-K+-ATPase activity and isoform adaptations to intense interval exercise and training in well-trained athletes.
Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation.
Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice.
Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death.
The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility.
Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue.
Subunit composition and role of Na+,K+-ATPases in ventricular myocytes.
Prolonged submaximal exercise induces isoform-specific Na+-K+-ATPase mRNA and protein responses in human skeletal muscle.
7-Dehydrocholesterol enhances ultraviolet A-induced oxidative stress in keratinocytes: roles of NADPH oxidase, mitochondria, and lipid rafts.
Calcium adaptation to sodium pump inhibition in a human megakaryocytic cell line.
Evidence that the H1-H2 domain of alpha1 subunit of (Na++K+)-ATPase participates in the regulation of cardiac contraction.
The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm.
Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism.
Quantification of alpha-subunit isoforms of Na,K-ATPase in rat resistance vessels.
The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart.
Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice.
Intense exercise up-regulates Na+,K+-ATPase isoform mRNA, but not protein expression in human skeletal muscle.
Stretch receptor-associated expression of alpha 3 isoform of the Na+, K+-ATPase in rat peripheral nervous system.
Vesicular localization and activity-dependent trafficking of presynaptic choline transporters.
Phospholemman expression in extraglomerular mesangium and afferent arteriole of the juxtaglomerular apparatus.
The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice.
The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice.
Na pump isoforms in human erythroid progenitor cells and mature erythrocytes.
Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1).
Altered Na,K-ATPase pattern in gamma-crystallin mutant mice.
Phorbol diacetate potentiates na(+)-k(+) ATPase inhibition by a putative endogenous ligand, marinobufagenin.
The influence of cycloheximide on Na,K-ATPase activity in cultured human lens epithelial cells.
Cellular depolarization of neurons in the locus ceruleus region of the guinea pig associated with the development of tolerance to opioids.
The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility.
Coupling of RYR1 and L-type calcium channels via calmodulin binding domains.
Immunocytochemical localization of NaK-ATPase isoforms in the rat and mouse ocular ciliary epithelium.
Quantification of the alpha(3) subunit of the Na(+)/K(+)-ATPase in developing rat cerebellum.
Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats.
Sodium pump inhibition and regional expression of sodium pump alpha-isoforms in lens.
Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart.
Regulation of Na+ pump function by aldosterone is alpha-subunit isoform specific.
Heterogeneity of Na+/K+-ATPase from rectal gland of Squalus acanthias is not due to alpha isoform diversity.
Isoforms of Na,K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures.
Isoform-specific monoclonal antibodies to Na,K-ATPase alpha subunits. Evidence for a tissue-specific post-translational modification of the alpha subunit.
Isoform-specific monoclonal antibodies to Na,K-ATPase alpha subunits. Evidence for a tissue-specific post-translational modification of the alpha subunit.
Williams D, Mehrabian M, Arshad H, Eid S, Sackmann C, Zhao W, Wang X, Ghodrati F, Verkuyl CE, Watts JC, Schmitt-Ulms G
PloS one 2021;16(11):e0258682
PloS one 2021;16(11):e0258682
Disposition of Proteins and Lipids in Synaptic Membrane Compartments Is Altered in Q175/Q7 Huntington's Disease Mouse Striatum.
Iuliano M, Seeley C, Sapp E, Jones EL, Martin C, Li X, DiFiglia M, Kegel-Gleason KB
Frontiers in synaptic neuroscience 2021;13:618391
Frontiers in synaptic neuroscience 2021;13:618391
An interaction between PRRT2 and Na(+)/K(+) ATPase contributes to the control of neuronal excitability.
Sterlini B, Romei A, Parodi C, Aprile D, Oneto M, Aperia A, Valente P, Valtorta F, Fassio A, Baldelli P, Benfenati F, Corradi A
Cell death & disease 2021 Mar 17;12(4):292
Cell death & disease 2021 Mar 17;12(4):292
Evaluation of the Efficacy of Dexamethasone-Eluting Electrode Array on the Post-Implant Cochlear Fibrotic Reaction by Three-Dimensional Immunofluorescence Analysis in Mongolian Gerbil Cochlea.
Toulemonde P, Risoud M, Lemesre PE, Beck C, Wattelet J, Tardivel M, Siepmann J, Vincent C
Journal of clinical medicine 2021 Jul 28;10(15)
Journal of clinical medicine 2021 Jul 28;10(15)
Differential Membrane Binding and Seeding of Distinct α-Synuclein Fibrillar Polymorphs.
Shrivastava AN, Bousset L, Renner M, Redeker V, Savistchenko J, Triller A, Melki R
Biophysical journal 2020 Mar 24;118(6):1301-1320
Biophysical journal 2020 Mar 24;118(6):1301-1320
Effects of testosterone suppression, hindlimb immobilization, and recovery on [(3)H]ouabain binding site content and Na(+), K(+)-ATPase isoforms in rat soleus muscle.
Altarawneh MM, Hanson ED, Betik AC, Petersen AC, Hayes A, McKenna MJ
Journal of applied physiology (Bethesda, Md. : 1985) 2020 Mar 1;128(3):501-513
Journal of applied physiology (Bethesda, Md. : 1985) 2020 Mar 1;128(3):501-513
Astrocyte deletion of α2-Na/K ATPase triggers episodic motor paralysis in mice via a metabolic pathway.
Smith SE, Chen X, Brier LM, Bumstead JR, Rensing NR, Ringel AE, Shin H, Oldenborg A, Crowley JR, Bice AR, Dikranian K, Ippolito JE, Haigis MC, Papouin T, Zhao G, Wong M, Culver JP, Bonni A
Nature communications 2020 Dec 2;11(1):6164
Nature communications 2020 Dec 2;11(1):6164
Resistance training upregulates skeletal muscle Na(+), K(+)-ATPase content, with elevations in both α(1) and α(2), but not β isoforms.
Altarawneh MM, Petersen AC, Farr T, Garnham A, Broatch JR, Halson S, Bishop DJ, McKenna MJ
European journal of applied physiology 2020 Aug;120(8):1777-1785
European journal of applied physiology 2020 Aug;120(8):1777-1785
Identification of the retinoschisin-binding site on the retinal Na/K-ATPase.
Plössl K, Straub K, Schmid V, Strunz F, Wild J, Merkl R, Weber BHF, Friedrich U
PloS one 2019;14(5):e0216320
PloS one 2019;14(5):e0216320
Characterization of transgenic mouse lines for labeling type I and type II afferent neurons in the cochlea.
Vyas P, Wu JS, Jimenez A, Glowatzki E, Fuchs PA
Scientific reports 2019 Apr 3;9(1):5549
Scientific reports 2019 Apr 3;9(1):5549
Proteomic Analysis of Dendritic Filopodia-Rich Fraction Isolated by Telencephalin and Vitronectin Interaction.
Furutani Y, Yoshihara Y
Frontiers in synaptic neuroscience 2018;10:27
Frontiers in synaptic neuroscience 2018;10:27
Differential Expression of Ca(2+)-buffering Protein Calretinin in Cochlear Afferent Fibers: A Possible Link to Vulnerability to Traumatic Noise.
Sharma K, Seo YW, Yi E
Experimental neurobiology 2018 Oct;27(5):397-407
Experimental neurobiology 2018 Oct;27(5):397-407
Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins.
Liebmann T, Fritz N, Kruusmägi M, Westin L, Bernhem K, Bondar A, Aperia A, Brismar H
International journal of molecular sciences 2018 Jul 29;19(8)
International journal of molecular sciences 2018 Jul 29;19(8)
Tyrosine Hydroxylase Expression in Type II Cochlear Afferents in Mice.
Vyas P, Wu JS, Zimmerman A, Fuchs P, Glowatzki E
Journal of the Association for Research in Otolaryngology : JARO 2017 Feb;18(1):139-151
Journal of the Association for Research in Otolaryngology : JARO 2017 Feb;18(1):139-151
Gestation changes sodium pump isoform expression, leading to changes in ouabain sensitivity, contractility, and intracellular calcium in rat uterus.
Floyd RV, Mobasheri A, Wray S
Physiological reports 2017 Dec;5(23)
Physiological reports 2017 Dec;5(23)
Retinoschisin is linked to retinal Na/K-ATPase signaling and localization.
Plössl K, Royer M, Bernklau S, Tavraz NN, Friedrich T, Wild J, Weber BHF, Friedrich U
Molecular biology of the cell 2017 Aug 1;28(16):2178-2189
Molecular biology of the cell 2017 Aug 1;28(16):2178-2189
Sodium pump organization in dendritic spines.
Blom H, Bernhem K, Brismar H
Neurophotonics 2016 Oct;3(4):041803
Neurophotonics 2016 Oct;3(4):041803
Dissociation between short-term unloading and resistance training effects on skeletal muscle Na+,K+-ATPase, muscle function, and fatigue in humans.
Perry BD, Wyckelsma VL, Murphy RM, Steward CH, Anderson M, Levinger I, Petersen AC, McKenna MJ
Journal of applied physiology (Bethesda, Md. : 1985) 2016 Nov 1;121(5):1074-1086
Journal of applied physiology (Bethesda, Md. : 1985) 2016 Nov 1;121(5):1074-1086
Cell specific differences in the protein abundances of GAPDH and Na(+),K(+)-ATPase in skeletal muscle from aged individuals.
Wyckelsma VL, McKenna MJ, Levinger I, Petersen AC, Lamboley CR, Murphy RM
Experimental gerontology 2016 Mar;75:8-15
Experimental gerontology 2016 Mar;75:8-15
Characterization of Fatty Acid Binding Protein 7 (FABP7) in the Murine Retina.
Su X, Tan QS, Parikh BH, Tan A, Mehta MN, Sia Wey Y, Tun SB, Li LJ, Han XY, Wong TY, Hunziker W, Luu CD, Owada Y, Barathi VA, Zhang SS, Chaurasia SS
Investigative ophthalmology & visual science 2016 Jun 1;57(7):3397-408
Investigative ophthalmology & visual science 2016 Jun 1;57(7):3397-408
Ouabain-Induced Signaling and Cell Survival in SK-N-SH Neuroblastoma Cells Differentiated by Retinoic Acid.
Akkuratov EE, Wu J, Sowa D, Shah ZA, Liu L
CNS & neurological disorders drug targets 2015;14(10):1343-9
CNS & neurological disorders drug targets 2015;14(10):1343-9
Single-fiber expression and fiber-specific adaptability to short-term intense exercise training of Na+-K+-ATPase α- and β-isoforms in human skeletal muscle.
Wyckelsma VL, McKenna MJ, Serpiello FR, Lamboley CR, Aughey RJ, Stepto NK, Bishop DJ, Murphy RM
Journal of applied physiology (Bethesda, Md. : 1985) 2015 Mar 15;118(6):699-706
Journal of applied physiology (Bethesda, Md. : 1985) 2015 Mar 15;118(6):699-706
The effects of knee injury on skeletal muscle function, Na+, K+-ATPase content, and isoform abundance.
Perry BD, Levinger P, Morris HG, Petersen AC, Garnham AP, Levinger I, McKenna MJ
Physiological reports 2015 Feb 1;3(2)
Physiological reports 2015 Feb 1;3(2)
Functional Interaction Between Na/K-ATPase and NMDA Receptor in Cerebellar Neurons.
Akkuratov EE, Lopacheva OM, Kruusmägi M, Lopachev AV, Shah ZA, Boldyrev AA, Liu L
Molecular neurobiology 2015 Dec;52(3):1726-1734
Molecular neurobiology 2015 Dec;52(3):1726-1734
Identification of a VxP Targeting Signal in the Flagellar Na+ /K+ -ATPase.
Laird JG, Pan Y, Modestou M, Yamaguchi DM, Song H, Sokolov M, Baker SA
Traffic (Copenhagen, Denmark) 2015 Dec;16(12):1239-53
Traffic (Copenhagen, Denmark) 2015 Dec;16(12):1239-53
Regional ion channel gene expression heterogeneity and ventricular fibrillation dynamics in human hearts.
Sivagangabalan G, Nazzari H, Bignolais O, Maguy A, Naud P, Farid T, Massé S, Gaborit N, Varro A, Nair K, Backx P, Vigmond E, Nattel S, Demolombe S, Nanthakumar K
PloS one 2014;9(1):e82179
PloS one 2014;9(1):e82179
Alternative splice isoforms of small conductance calcium-activated SK2 channels differ in molecular interactions and surface levels.
Scholl ES, Pirone A, Cox DH, Duncan RK, Jacob MH
Channels (Austin, Tex.) 2014;8(1):62-75
Channels (Austin, Tex.) 2014;8(1):62-75
Distribution of Na,K-ATPase α subunits in rat vestibular sensory epithelia.
Schuth O, McLean WJ, Eatock RA, Pyott SJ
Journal of the Association for Research in Otolaryngology : JARO 2014 Oct;15(5):739-54
Journal of the Association for Research in Otolaryngology : JARO 2014 Oct;15(5):739-54
Numb regulates the polarized delivery of cyclic nucleotide-gated ion channels in rod photoreceptor cilia.
Ramamurthy V, Jolicoeur C, Koutroumbas D, Mühlhans J, Le YZ, Hauswirth WW, Giessl A, Cayouette M
The Journal of neuroscience : the official journal of the Society for Neuroscience 2014 Oct 15;34(42):13976-87
The Journal of neuroscience : the official journal of the Society for Neuroscience 2014 Oct 15;34(42):13976-87
Agonist-dependent and -independent dopamine-1-like receptor signalling differentially regulates downstream effectors.
Roosterman D
The FEBS journal 2014 Nov;281(21):4792-804
The FEBS journal 2014 Nov;281(21):4792-804
Regulation and pharmacological blockade of sodium-potassium ATPase: a novel pathway to neuropathy.
Paul D, Soignier RD, Minor L, Tau H, Songu-Mize E, Gould HJ 3rd
Journal of the neurological sciences 2014 May 15;340(1-2):139-43
Journal of the neurological sciences 2014 May 15;340(1-2):139-43
The effects of osteoarthritis and age on skeletal muscle strength, Na+-K+-ATPase content, gene and isoform expression.
Perry BD, Levinger P, Serpiello FR, Caldow MK, Cameron-Smith D, Bartlett JR, Feller JA, Bergman NR, Levinger I, McKenna MJ
Journal of applied physiology (Bethesda, Md. : 1985) 2013 Nov;115(10):1443-9
Journal of applied physiology (Bethesda, Md. : 1985) 2013 Nov;115(10):1443-9
Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons.
Edwards IJ, Bruce G, Lawrenson C, Howe L, Clapcote SJ, Deuchars SA, Deuchars J
The Journal of neuroscience : the official journal of the Society for Neuroscience 2013 Jun 12;33(24):9913-9
The Journal of neuroscience : the official journal of the Society for Neuroscience 2013 Jun 12;33(24):9913-9
Overexpression of the polycystin-1 C-tail enhances sensitivity of M-1 cells to ouabain.
Jansson K, Magenheimer BS, Maser RL, Calvet JP, Blanco G
The Journal of membrane biology 2013 Jul;246(7):581-90
The Journal of membrane biology 2013 Jul;246(7):581-90
A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3.
Azarias G, Kruusmägi M, Connor S, Akkuratov EE, Liu XL, Lyons D, Brismar H, Broberger C, Aperia A
The Journal of biological chemistry 2013 Jan 25;288(4):2734-43
The Journal of biological chemistry 2013 Jan 25;288(4):2734-43
Cardiac glycosides block cancer growth through HIF-1α- and NF-κB-mediated Plk1.
Xie CM, Liu XY, Yu S, Cheng CH
Carcinogenesis 2013 Aug;34(8):1870-80
Carcinogenesis 2013 Aug;34(8):1870-80
Unchanged [3H]ouabain binding site content but reduced Na+-K+ pump α2-protein abundance in skeletal muscle in older adults.
McKenna MJ, Perry BD, Serpiello FR, Caldow MK, Levinger P, Cameron-Smith D, Levinger I
Journal of applied physiology (Bethesda, Md. : 1985) 2012 Nov;113(10):1505-11
Journal of applied physiology (Bethesda, Md. : 1985) 2012 Nov;113(10):1505-11
Impaired exercise performance and muscle Na(+),K(+)-pump activity in renal transplantation and haemodialysis patients.
Petersen AC, Leikis MJ, McMahon LP, Kent AB, Murphy KT, Gong X, McKenna MJ
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2012 May;27(5):2036-43
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2012 May;27(5):2036-43
Nearest neighbor analysis of dopamine D1 receptors and Na(+)-K(+)-ATPases in dendritic spines dissected by STED microscopy.
Blom H, Rönnlund D, Scott L, Spicarova Z, Rantanen V, Widengren J, Aperia A, Brismar H
Microscopy research and technique 2012 Feb;75(2):220-8
Microscopy research and technique 2012 Feb;75(2):220-8
mGluR6 deletion renders the TRPM1 channel in retina inactive.
Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T, Vardi N
Journal of neurophysiology 2012 Feb;107(3):948-57
Journal of neurophysiology 2012 Feb;107(3):948-57
Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor.
Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ, Roman RJ, Harder DR, Koehler RC
Journal of neurochemistry 2012 Apr;121(1):168-79
Journal of neurochemistry 2012 Apr;121(1):168-79
In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models.
Dunn DE, He DN, Yang P, Johansen M, Newman RA, Lo DC
Journal of neurochemistry 2011 Nov;119(4):805-14
Journal of neurochemistry 2011 Nov;119(4):805-14
Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2.
Leo L, Gherardini L, Barone V, De Fusco M, Pietrobon D, Pizzorusso T, Casari G
PLoS genetics 2011 Jun;7(6):e1002129
PLoS genetics 2011 Jun;7(6):e1002129
Spatial distribution of Na+-K+-ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy.
Blom H, Rönnlund D, Scott L, Spicarova Z, Widengren J, Bondar A, Aperia A, Brismar H
BMC neuroscience 2011 Jan 27;12:16
BMC neuroscience 2011 Jan 27;12:16
Aldosterone increases Na+ -K+ -ATPase activity in skeletal muscle of patients with Conn's syndrome.
Phakdeekitcharoen B, Kittikanokrat W, Kijkunasathian C, Chatsudthipong V
Clinical endocrinology 2011 Feb;74(2):152-9
Clinical endocrinology 2011 Feb;74(2):152-9
Changes in Na+, K+-ATPase activity and alpha 3 subunit expression in CNS after administration of Na+, K+-ATPase inhibitors.
Bersier MG, Peña C, Arnaiz GR
Neurochemical research 2011 Feb;36(2):297-303
Neurochemical research 2011 Feb;36(2):297-303
Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts.
Quintas LE, Pierre SV, Liu L, Bai Y, Liu X, Xie ZJ
Journal of molecular and cellular cardiology 2010 Sep;49(3):525-31
Journal of molecular and cellular cardiology 2010 Sep;49(3):525-31
BK channels mediate cholinergic inhibition of high frequency cochlear hair cells.
Wersinger E, McLean WJ, Fuchs PA, Pyott SJ
PloS one 2010 Nov 4;5(11):e13836
PloS one 2010 Nov 4;5(11):e13836
Immunostaining for the α3 isoform of the Na+/K+-ATPase is selective for functionally identified muscle spindle afferents in vivo.
Parekh A, Campbell AJ, Djouhri L, Fang X, McMullan S, Berry C, Acosta C, Lawson SN
The Journal of physiology 2010 Nov 1;588(Pt 21):4131-43
The Journal of physiology 2010 Nov 1;588(Pt 21):4131-43
Increase in ouabain-sensitive K+-ATPase activity in hepatocellular carcinoma by overexpression of Na+, K+-ATPase alpha 3-isoform.
Shibuya K, Fukuoka J, Fujii T, Shimoda E, Shimizu T, Sakai H, Tsukada K
European journal of pharmacology 2010 Jul 25;638(1-3):42-6
European journal of pharmacology 2010 Jul 25;638(1-3):42-6
Mutant huntingtin and glycogen synthase kinase 3-beta accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington's disease.
Valencia A, Reeves PB, Sapp E, Li X, Alexander J, Kegel KB, Chase K, Aronin N, DiFiglia M
Journal of neuroscience research 2010 Jan;88(1):179-90
Journal of neuroscience research 2010 Jan;88(1):179-90
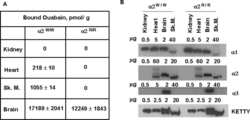
The ouabain-binding site of the α2 isoform of Na,K-ATPase plays a role in blood pressure regulation during pregnancy.
Oshiro N, Dostanic-Larson I, Neumann JC, Lingrel JB
American journal of hypertension 2010 Dec;23(12):1279-85
American journal of hypertension 2010 Dec;23(12):1279-85
Enhanced pressor response to increased CSF sodium concentration and to central ANG I in heterozygous alpha2 Na+ -K+ -ATPase knockout mice.
Hou X, Theriault SF, Dostanic-Larson I, Moseley AE, Lingrel JB, Wu H, Dean S, Van Huysse JW
American journal of physiology. Regulatory, integrative and comparative physiology 2009 May;296(5):R1427-38
American journal of physiology. Regulatory, integrative and comparative physiology 2009 May;296(5):R1427-38
Distribution of the Na,K-ATPase alpha subunit in the rat spiral ganglion and organ of corti.
McLean WJ, Smith KA, Glowatzki E, Pyott SJ
Journal of the Association for Research in Otolaryngology : JARO 2009 Mar;10(1):37-49
Journal of the Association for Research in Otolaryngology : JARO 2009 Mar;10(1):37-49
Glutamate transporter coupling to Na,K-ATPase.
Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR
The Journal of neuroscience : the official journal of the Society for Neuroscience 2009 Jun 24;29(25):8143-55
The Journal of neuroscience : the official journal of the Society for Neuroscience 2009 Jun 24;29(25):8143-55
Ca2+ influx mechanisms in caveolae vesicles of pulmonary smooth muscle plasma membrane under inhibition of alpha2beta1 isozyme of Na+/K+-ATPase by ouabain.
Ghosh B, Kar P, Mandal A, Dey K, Chakraborti T, Chakraborti S
Life sciences 2009 Jan 30;84(5-6):139-48
Life sciences 2009 Jan 30;84(5-6):139-48
Over-expression of translationally controlled tumor protein in lens epithelial cells seems to be associated with cataract development.
Kim MJ, Lyu J, Sohn KB, Kim M, Cho MC, Joo CK, Lee K
Transgenic research 2009 Dec;18(6):953-60
Transgenic research 2009 Dec;18(6):953-60
Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries.
Dora KA, Gallagher NT, McNeish A, Garland CJ
Circulation research 2008 May 23;102(10):1247-55
Circulation research 2008 May 23;102(10):1247-55
Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex.
Molday LL, Wu WW, Molday RS
The Journal of biological chemistry 2007 Nov 9;282(45):32792-801
The Journal of biological chemistry 2007 Nov 9;282(45):32792-801
Muscle Na+-K+-ATPase activity and isoform adaptations to intense interval exercise and training in well-trained athletes.
Aughey RJ, Murphy KT, Clark SA, Garnham AP, Snow RJ, Cameron-Smith D, Hawley JA, McKenna MJ
Journal of applied physiology (Bethesda, Md. : 1985) 2007 Jul;103(1):39-47
Journal of applied physiology (Bethesda, Md. : 1985) 2007 Jul;103(1):39-47
Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation.
Nguyen AN, Wallace DP, Blanco G
Journal of the American Society of Nephrology : JASN 2007 Jan;18(1):46-57
Journal of the American Society of Nephrology : JASN 2007 Jan;18(1):46-57
Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice.
Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Jan 17;27(3):616-26
The Journal of neuroscience : the official journal of the Society for Neuroscience 2007 Jan 17;27(3):616-26
Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death.
Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC
The Journal of comparative neurology 2007 Jan 1;500(1):20-46
The Journal of comparative neurology 2007 Jan 1;500(1):20-46
The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility.
Sanchez G, Nguyen AN, Timmerberg B, Tash JS, Blanco G
Molecular human reproduction 2006 Sep;12(9):565-76
Molecular human reproduction 2006 Sep;12(9):565-76
Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue.
Lunde PK, Sejersted OM, Thorud HM, Tønnessen T, Henriksen UL, Christensen G, Westerblad H, Bruton J
Circulation research 2006 Jun 23;98(12):1514-9
Circulation research 2006 Jun 23;98(12):1514-9
Subunit composition and role of Na+,K+-ATPases in ventricular myocytes.
Harada K, Lin H, Endo Y, Fujishiro N, Sakamoto Y, Inoue M
The journal of physiological sciences : JPS 2006 Feb;56(1):113-21
The journal of physiological sciences : JPS 2006 Feb;56(1):113-21
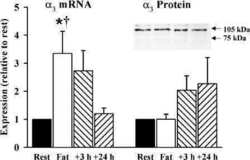
Prolonged submaximal exercise induces isoform-specific Na+-K+-ATPase mRNA and protein responses in human skeletal muscle.
Murphy KT, Petersen AC, Goodman C, Gong X, Leppik JA, Garnham AP, Cameron-Smith D, Snow RJ, McKenna MJ
American journal of physiology. Regulatory, integrative and comparative physiology 2006 Feb;290(2):R414-24
American journal of physiology. Regulatory, integrative and comparative physiology 2006 Feb;290(2):R414-24
7-Dehydrocholesterol enhances ultraviolet A-induced oxidative stress in keratinocytes: roles of NADPH oxidase, mitochondria, and lipid rafts.
Valencia A, Rajadurai A, Carle AB, Kochevar IE
Free radical biology & medicine 2006 Dec 1;41(11):1704-18
Free radical biology & medicine 2006 Dec 1;41(11):1704-18
Calcium adaptation to sodium pump inhibition in a human megakaryocytic cell line.
Kimura M, Cao X, Aviv A
American journal of physiology. Cell physiology 2005 Oct;289(4):C891-7
American journal of physiology. Cell physiology 2005 Oct;289(4):C891-7
Evidence that the H1-H2 domain of alpha1 subunit of (Na++K+)-ATPase participates in the regulation of cardiac contraction.
Xu KY, Takimoto E, Juang GJ, Zhang Q, Rohde H, Myers AC
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2005 Jan;19(1):53-61
FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2005 Jan;19(1):53-61
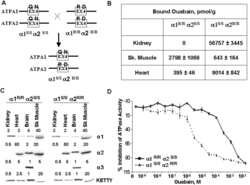
The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm.
Radzyukevich TL, Moseley AE, Shelly DA, Redden GA, Behbehani MM, Lingrel JB, Paul RJ, Heiny JA
American journal of physiology. Cell physiology 2004 Nov;287(5):C1300-10
American journal of physiology. Cell physiology 2004 Nov;287(5):C1300-10
Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism.
de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ
Neuron 2004 Jul 22;43(2):169-75
Neuron 2004 Jul 22;43(2):169-75
Quantification of alpha-subunit isoforms of Na,K-ATPase in rat resistance vessels.
Hansen O
Acta physiologica Scandinavica 2004 Jan;180(1):49-56
Acta physiologica Scandinavica 2004 Jan;180(1):49-56
The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart.
Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB
The Journal of biological chemistry 2004 Dec 24;279(52):54053-61
The Journal of biological chemistry 2004 Dec 24;279(52):54053-61
Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice.
Shelly DA, He S, Moseley A, Weber C, Stegemeyer M, Lynch RM, Lingrel J, Paul RJ
American journal of physiology. Cell physiology 2004 Apr;286(4):C813-20
American journal of physiology. Cell physiology 2004 Apr;286(4):C813-20
Intense exercise up-regulates Na+,K+-ATPase isoform mRNA, but not protein expression in human skeletal muscle.
Murphy KT, Snow RJ, Petersen AC, Murphy RM, Mollica J, Lee JS, Garnham AP, Aughey RJ, Leppik JA, Medved I, Cameron-Smith D, McKenna MJ
The Journal of physiology 2004 Apr 15;556(Pt 2):507-19
The Journal of physiology 2004 Apr 15;556(Pt 2):507-19
Stretch receptor-associated expression of alpha 3 isoform of the Na+, K+-ATPase in rat peripheral nervous system.
Dobretsov M, Hastings SL, Sims TJ, Stimers JR, Romanovsky D
Neuroscience 2003;116(4):1069-80
Neuroscience 2003;116(4):1069-80
Vesicular localization and activity-dependent trafficking of presynaptic choline transporters.
Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD
The Journal of neuroscience : the official journal of the Society for Neuroscience 2003 Oct 29;23(30):9697-709
The Journal of neuroscience : the official journal of the Society for Neuroscience 2003 Oct 29;23(30):9697-709
Phospholemman expression in extraglomerular mesangium and afferent arteriole of the juxtaglomerular apparatus.
Wetzel RK, Sweadner KJ
American journal of physiology. Renal physiology 2003 Jul;285(1):F121-9
American journal of physiology. Renal physiology 2003 Jul;285(1):F121-9
The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice.
Moseley AE, Lieske SP, Wetzel RK, James PF, He S, Shelly DA, Paul RJ, Boivin GP, Witte DP, Ramirez JM, Sweadner KJ, Lingrel JB
The Journal of biological chemistry 2003 Feb 14;278(7):5317-24
The Journal of biological chemistry 2003 Feb 14;278(7):5317-24
The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice.
Dostanic I, Lorenz JN, Schultz Jel J, Grupp IL, Neumann JC, Wani MA, Lingrel JB
The Journal of biological chemistry 2003 Dec 26;278(52):53026-34
The Journal of biological chemistry 2003 Dec 26;278(52):53026-34
Na pump isoforms in human erythroid progenitor cells and mature erythrocytes.
Hoffman JF, Wickrema A, Potapova O, Milanick M, Yingst DR
Proceedings of the National Academy of Sciences of the United States of America 2002 Oct 29;99(22):14572-7
Proceedings of the National Academy of Sciences of the United States of America 2002 Oct 29;99(22):14572-7
Ubiquitin modification of serum and glucocorticoid-induced protein kinase-1 (SGK-1).
Brickley DR, Mikosz CA, Hagan CR, Conzen SD
The Journal of biological chemistry 2002 Nov 8;277(45):43064-70
The Journal of biological chemistry 2002 Nov 8;277(45):43064-70
Altered Na,K-ATPase pattern in gamma-crystallin mutant mice.
Moseley A, Graw J, Delamere NA
Investigative ophthalmology & visual science 2002 May;43(5):1517-9
Investigative ophthalmology & visual science 2002 May;43(5):1517-9
Phorbol diacetate potentiates na(+)-k(+) ATPase inhibition by a putative endogenous ligand, marinobufagenin.
Fedorova OV, Dorofeeva NA, Lopatin DA, Lakatta EG, Bagrov AY
Hypertension (Dallas, Tex. : 1979) 2002 Feb;39(2):298-302
Hypertension (Dallas, Tex. : 1979) 2002 Feb;39(2):298-302
The influence of cycloheximide on Na,K-ATPase activity in cultured human lens epithelial cells.
Cui G, Dean WL, Delamere NA
Investigative ophthalmology & visual science 2002 Aug;43(8):2714-20
Investigative ophthalmology & visual science 2002 Aug;43(8):2714-20
Cellular depolarization of neurons in the locus ceruleus region of the guinea pig associated with the development of tolerance to opioids.
Kong JQ, Meng J, Biser PS, Fleming WW, Taylor DA
The Journal of pharmacology and experimental therapeutics 2001 Sep;298(3):909-16
The Journal of pharmacology and experimental therapeutics 2001 Sep;298(3):909-16
The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility.
He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB
American journal of physiology. Regulatory, integrative and comparative physiology 2001 Sep;281(3):R917-25
American journal of physiology. Regulatory, integrative and comparative physiology 2001 Sep;281(3):R917-25
Coupling of RYR1 and L-type calcium channels via calmodulin binding domains.
Sencer S, Papineni RV, Halling DB, Pate P, Krol J, Zhang JZ, Hamilton SL
The Journal of biological chemistry 2001 Oct 12;276(41):38237-41
The Journal of biological chemistry 2001 Oct 12;276(41):38237-41
Immunocytochemical localization of NaK-ATPase isoforms in the rat and mouse ocular ciliary epithelium.
Wetzel RK, Sweadner KJ
Investigative ophthalmology & visual science 2001 Mar;42(3):763-9
Investigative ophthalmology & visual science 2001 Mar;42(3):763-9
Quantification of the alpha(3) subunit of the Na(+)/K(+)-ATPase in developing rat cerebellum.
Biser PS, Thayne KA, Kong JQ, Fleming WW, Taylor DA
Brain research. Developmental brain research 2000 Oct 28;123(2):165-72
Brain research. Developmental brain research 2000 Oct 28;123(2):165-72
Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats.
Fedorova OV, Lakatta EG, Bagrov AY
Circulation 2000 Dec 12;102(24):3009-14
Circulation 2000 Dec 12;102(24):3009-14
Sodium pump inhibition and regional expression of sodium pump alpha-isoforms in lens.
Tao QF, Hollenberg NK, Graves SW
Hypertension (Dallas, Tex. : 1979) 1999 Nov;34(5):1168-74
Hypertension (Dallas, Tex. : 1979) 1999 Nov;34(5):1168-74
Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart.
James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB
Molecular cell 1999 May;3(5):555-63
Molecular cell 1999 May;3(5):555-63
Regulation of Na+ pump function by aldosterone is alpha-subunit isoform specific.
Pfeiffer R, Beron J, Verrey F
The Journal of physiology 1999 May 1;516 ( Pt 3)(Pt 3):647-55
The Journal of physiology 1999 May 1;516 ( Pt 3)(Pt 3):647-55
Heterogeneity of Na+/K+-ATPase from rectal gland of Squalus acanthias is not due to alpha isoform diversity.
Hansen O
Pflugers Archiv : European journal of physiology 1999 Mar;437(4):517-22
Pflugers Archiv : European journal of physiology 1999 Mar;437(4):517-22
Isoforms of Na,K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures.
Peng L, Martin-Vasallo P, Sweadner KJ
The Journal of neuroscience : the official journal of the Society for Neuroscience 1997 May 15;17(10):3488-502
The Journal of neuroscience : the official journal of the Society for Neuroscience 1997 May 15;17(10):3488-502
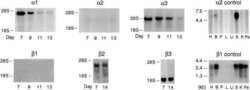
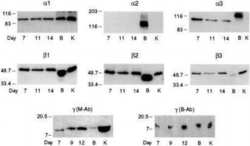
Isoform-specific monoclonal antibodies to Na,K-ATPase alpha subunits. Evidence for a tissue-specific post-translational modification of the alpha subunit.
Arystarkhova E, Sweadner KJ
The Journal of biological chemistry 1996 Sep 20;271(38):23407-17
The Journal of biological chemistry 1996 Sep 20;271(38):23407-17
Isoform-specific monoclonal antibodies to Na,K-ATPase alpha subunits. Evidence for a tissue-specific post-translational modification of the alpha subunit.
Arystarkhova E, Sweadner KJ
The Journal of biological chemistry 1996 Sep 20;271(38):23407-17
The Journal of biological chemistry 1996 Sep 20;271(38):23407-17
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
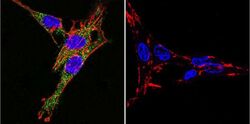
- Main image
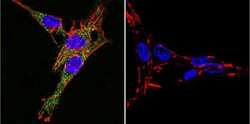
- Experimental details
- Immunofluorescent analysis of Sodiµm/Potassium ATPase alpha-3 using Sodiµm/Potassium ATPase alpha-3 Monoclonal antibody (XVIF9-G10) (Product # MA3-915) shows staining in C6 glioma cells. Sodiµm/Potassium ATPase alpha-3 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
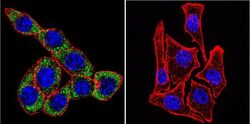
- Main image
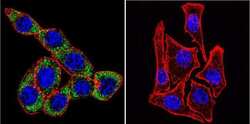
- Experimental details
- Immunofluorescent analysis of Sodiµm/Potassium ATPase alpha-3 using Sodiµm/Potassium ATPase alpha-3 Monoclonal antibody (XVIF9-G10) (Product # MA3-915) shows staining in HeLa cells. Sodiµm/Potassium ATPase alpha-3 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
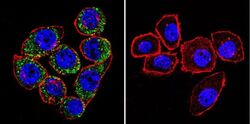
- Main image
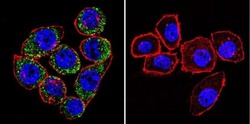
- Experimental details
- Immunofluorescent analysis of Sodiµm/Potassium ATPase alpha-3 using Sodiµm/Potassium ATPase alpha-3 Monoclonal antibody (XVIF9-G10) (Product # MA3-915) shows staining in U251 glioma cells. Sodiµm/Potassium ATPase alpha-3 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
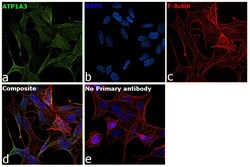
- Main image
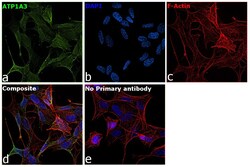
- Experimental details
- Immunofluorescence analysis of ATP1A3 was performed using SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with ATP1A3 Monoclonal Antibody (XVIF9-G10) (Product # MA3-915) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing membrane localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of Sodiµm/Potassium ATPase alpha-3 using Sodiµm/Potassium ATPase alpha-3 Monoclonal antibody (XVIF9-G10) (Product # MA3-915) shows staining in C6 glioma cells. Sodiµm/Potassium ATPase alpha-3 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of Sodiµm/Potassium ATPase alpha-3 using Sodiµm/Potassium ATPase alpha-3 Monoclonal antibody (XVIF9-G10) (Product # MA3-915) shows staining in HeLa cells. Sodiµm/Potassium ATPase alpha-3 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescent analysis of Sodiµm/Potassium ATPase alpha-3 using Sodiµm/Potassium ATPase alpha-3 Monoclonal antibody (XVIF9-G10) (Product # MA3-915) shows staining in U251 glioma cells. Sodiµm/Potassium ATPase alpha-3 staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) at a dilution of 1:20 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product # 35552 for GAR, Product # 35503 for GAM). Images were taken at 60X magnification.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Immunofluorescence analysis of ATP1A3 was performed using SH-SY5Y cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 15 minutes, and blocked with 2% BSA for 45 minutes at room temperature. The cells were labeled with ATP1A3 Monoclonal Antibody (XVIF9-G10) (Product # MA3-915) at 5 µg/mL in 0.1% BSA, incubated at 4 degree celsius overnight and then labeled with Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 (Product # A32723), (1:2000), for 45 minutes at room temperature (Panel a: Green). Nuclei (Panel b:Blue) were stained with ProLong™ Diamond Antifade Mountant with DAPI (Product # P36962). F-actin (Panel c: Red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing membrane localization. Panel e represents control cells with no primary antibody to assess background. The images were captured at 60X magnification.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
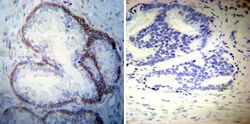
- Experimental details
- Immunohistochemistry was performed on cancer biopsies of deparaffinized Human prostate carcinoma tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a mouse monoclonal antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
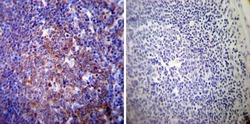
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human tonsil tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
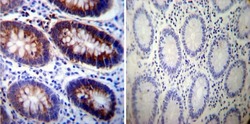
- Experimental details
- Immunohistochemistry was performed on normal deparaffinized Human colon tissue tissues. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:200 with a mouse monoclonal antibody recognizing Sodiµm/Potassium ATPase alpha-3 (Product # MA3-915) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
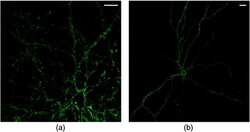
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
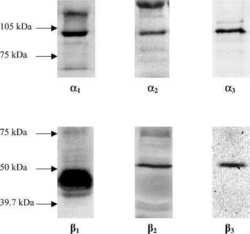
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
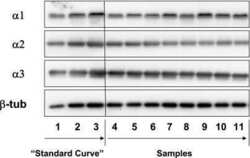
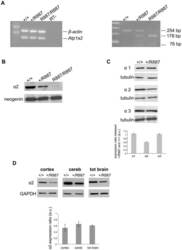
- Figure 2 In vivo expression of mutant Atp1a2 . Total RNA and protein samples were isolated from brain of wild type (+/+), Atp1a2 +/R887 (+/R887) and homozygous Atp1a2 R887/R887 (R887/R887) mice at E19.5. A. Left panel. Semi quantitative Atp1a2 RT-PCR (254 bp fragment) on brain cDNA. The b-actin fragment (610 bp) is included as in-tube normalizer. Right panel, same Atp1a2 RT-PCR fragments digested by MspI. B. Protein blot of microsomal fraction probed with anti-alpha2 Na,K-ATPase antibody and anti-neogenin as loading control; the alpha2 Na,K-ATPase and neogenin bands appear at the expected size of 110 kDa and 52 kDa, respectively. C. Total brain lysates from adult wild type and Atp1a2 +/R887 mice probed with anti-alpha1, alpha2, and alpha3 Na,K-ATPase antibodies; anti- tubulin as loading control. Densitometric quantization shows a 50% reduction of the heterozygous mutant alpha2 level compared to wild type. Error bars represent +- SD; Student's t test p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
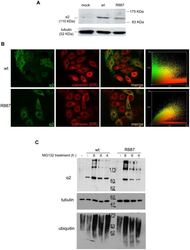
- Figure 3 In vitro expression and localization of alpha2-ATPase . A. Immunoblot of HeLa cells transfected with pA2-wt-Myc (wt) and pA2-R887-Myc (R887) probed with anti alpha2-ATPase antibody. B. Calnexin (red) and alpha2 ATPase (probed with anti-myc; green) immunofluorescence staining of wt or R887 transfected Hela cells. R887 alpha2 ATPase mostly colocalises with the endoplasmic reticulum. In the right graphs, the scatter plot of red (ch1) vs. green (ch2) colocalization intensities. (wt parameters: Rr 0.369, R 0.6, Ch1:Ch2 0.999; R887 parameters: Rr 0.558, R 0.755, Ch1:Ch2 0.997). C. HeLa cells expressing wt or R887 variant of alpha2 ATPase are treated with the reversible proteasome inhibitor MG132 (10 uM for 4, 6 and 8 hrs). Blots are probed with anti- alpha2 isoform antibody, anti-ubiquitin antibody and anti-tubulin as loading control.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
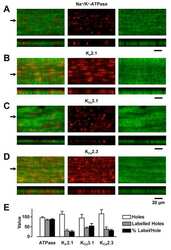
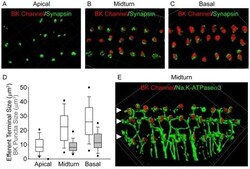
- Figure 4 Tonotopic distribution of BK channels in outer hair cells. 3D renderings of confocal z-stacks of apical (A), middle (B), and basal (C) cochlear turns immunostained with a monoclonal antibody against the BK channel (red) and a polyclonal antibody against synapsin (green) to label the efferent presynaptic terminals show that BK channel immunoreactivity in the three rows of outer hair cells (when present) is associated with efferent terminals. (D) Moreover, the size of BK channel immunopuncta increases from apical to middle and basal turns, paralleling an increase in size and likely number of efferent terminals. Data are presented as box plots representing the median (box interior), the 25th and 75th percentile (box boundaries), the 10th and 90th percentile (whiskers), and the 5th and 95th percentile (dots). (E) 3D renderings of confocal z-stacks of a middle cochlear turn immunostained with a polyclonal antibody against the BK channel (red) and a monoclonal antibody against the NaK-ATPase alpha3 (green) to label the efferent presynaptic terminal membrane shows adjacent but not co localized expression of the BK channel with the presynaptic efferent terminal membrane. The location of the three rows of outer hair cells are indicated (solid arrowheads). Grid dimensions equal 10 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
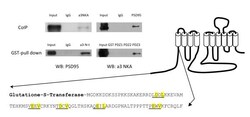
- Experimental details
- Figure 1 Co-immunoprecipitation and GST pull down assay of PSD-95 and alpha3 NKA . Co-immunoprecipitation (CoIP) shows interaction between PSD-95 and alpha3 NKA. GST pull down assays shows interaction of PSD-95 with the alpha3 NKA N-terminus and a specific PDZ3 interaction.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
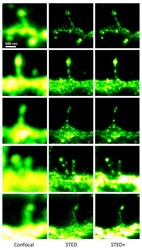
- Experimental details
- Figure 3 STED microscopy dissecting the localization of Na + ,K + -ATPase in cultured striatal neurons . The 5 x 3 mosaic shows a comparision of the confocal (left) and STED images (middle) of the Alexa-594 immunolabelled alpha3 NKA in dendritic spines. Postprocessing of the raw STED data by a Richardson-Lucy deconvolution further enhances the details as shown in the right row of images. Scale bar: 500 nm
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
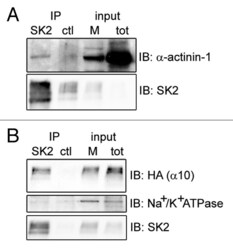
- Experimental details
- Figure 2. alpha-actinin-1 and alpha9/10-nAChRs interact with SK2 channels in Xenopus oocytes. Immunoblots show co-precipitation of exogenous SK2 with co-expressed alpha-actinin-1 ( A ) and HA-tagged alpha9/10-nAChRs ( B ) in Xenopus laevis oocytes. As negative controls, alpha-actinin-1 and nAChRs did not co-precipitate with an unrelated antibody of the same IgG subclass (anti-mGluR5; ctl lane in A and B ) and SK2 did not co-precipitate with endogenous Na + /K + ATPase ( B , lower panel). Input, 6% of total membrane fraction (M) and lysate (tot) for all panels. n = 4 separate experiments.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
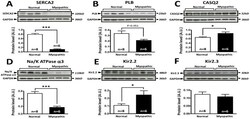
- Experimental details
- Figure 5 Comparison of protein expression in LV sample from control and cardiomyopathic patients. A: Top; representative SERCA2 and respective GAPDH stainings obtained in normal and cardiomyopathic LV samples. Bottom; Mean+-SEM, *** P
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
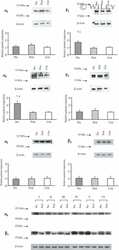
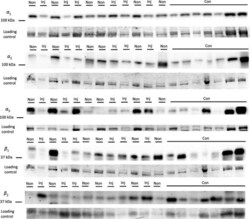
- Figure 4 Representative western blot images for each NKA isoform analyzed ( alpha 1-3 and beta 1-2 ), including example of the coomassie stain used to normalize blot density to the total protein content of the sample. ""Inj"" represents the injured leg from the KI group, ""Non"" represents the noninjured leg from the KI group, and ""CON"" represents the control group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
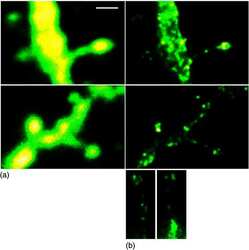
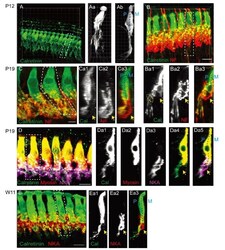
- Fig. 1 Differential trajectories of the cochlear afferent fibers (CAFs) in the organ of Corti. (A) A three-dimensional (3D) reconstructed image of the organ of Corti immunolabeled with anti-calretinin in a P12 rat. (Aa~Ab) XY and ZY projection views of the marked area in A. (B) 3D-reconstructed images of an organ of Corti in a P11 rat that is double-immunolabeled with anti-calretinin (green) and anti-neurofilament (NF; red). (Ba1~Ba3) ZY section views of the marked area in B. An approximate location of habenular perforata is marked with dotted oval. (C) A 3D reconstructed image of an organ of Corti from a P19 rat that was double-immunolabeled with anti-calretinin (green) and anti-NF (red). (Ca1~Ca3) ZY projection views of the marked area in C. (D) A 3D reconstructed image of an organ of Corti from a P19 rat that was triple-immunolabeled with anti-calretinin (green), anti-myosin (red) and anti-NKA (magenta). (Da1~Da5) ZY section views of the marked area in D. (E) A 3D reconstructed image of an organ of Corti from a 11-week-old rat (W11) that was double-immunolabeled with anti-calretinin (green) and anti-Na, K-ATPase alpha 3 (NKA; red). (Ea1~Ea3) ZY projection view of the marked area in E. At all ages examined, calretinin-rich and calretinin-poor fibers (arrow) exhibit segregated courses. The pillar (P) and modiolar side (M) of IHC is marked with dotted lines (Ab, Ba3, Ca3, Da5, and Ea3).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
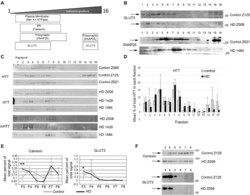
- FIGURE 7 Subcellular fractionation by density gradient ultracentrifugation of human putamen. Dorsal putamen was fractionated as described in section ""Materials and Methods."" Fractions were assessed by SDS-PAGE and western blot analysis using different cell compartment markers. Fractions 1-16 were removed from top to bottom. Equal volumes of each fraction were loaded per lane. (A) Diagram at left shows approximate distribution of proteins representative of different subcellular compartments in the control putamen. (B) Shown are representative images of western blots detecting GLUT3 and SNAP25 in control and HD putamen. (C) Shown are WB images of Huntingtin (HTT) and mutant HTT (mHTT) signals in 16 fractions from all human putamen samples. mHTT was detected with 1C2 which recognizes the expanded polyQ region in mutant HTT but not HTT in control samples. (D) Bar graph shows mean percent +- SD of HTT signal obtained with antibody Ab1 in control and HD human putamen in each of 16 fractions as a percent of total HTT signal summed across all fractions. There was no significant difference in HTT signal between control and HD in any of the fractions ( N = 3). (E) Fractions 3-8 were analyzed in pairs and run on the same gel to eliminate variability due to different runs. These fractions were chosen because most proteins tested had peaks in this range. Seven probes were analyzed by western blot for each gel (Calnexin, GLUT3, Na+/K+ ATPase, NMDAR 2b, PSD95, SNAP25, and XK). Band intens
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
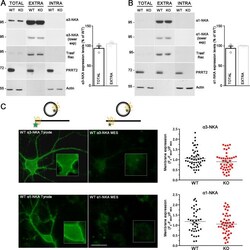
- Fig. 1 PRRT2 interacts with alpha3-NKA and alpha1-NKA subunits. A Pulldown of alpha3-NKA and alpha1-NKA by PRRT2 : Affinity-purified PRRT2-HA or BAP-HA were incubated with total mouse brain extracts. After pulldown (PD), pellets were solubilized and subjected to western blotting with alpha3-NKA and alpha1-NKA antibodies. Mouse brain lysates incubated with PRRT2-HA showed specific immunoreactivity for alpha3-NKA and alpha1-NKA in the precipitates, which were not detected with BAP-HA. Top: Representative immunoblots are shown. Bottom: Quantification of the alpha3-NKA and alpha1-NKA immunoreactivities in the pulled down samples normalized to the BAP-HA control (means +- SEM, n = 4 independent experiments, ** p < 0.01; unpaired Student's t -test). Input, 10 mug total extract. B Co-immunoprecipitation of PRRT2 with alpha3-NKA and alpha1-NKA antibodies : Detergent extracts of mouse brain were immunoprecipitated (IP) with monoclonal antibodies (mAbs) to alpha3-NKA, alpha1-NKA or GFP (used as a control), as indicated. After the electrophoretic separation of the immunocomplexes and western blotting, membranes were probed with alpha3-NKA or alpha1-NKA antibodies to test the immunoprecipitation efficiency, as well as with polyclonal PRRT2 antibodies to test for co-immunoprecipitation. Left: Representative immunoblots are shown. Right: Quantification of the PRRT2 immunoreactive signal in the immunoprecipitated samples normalized to the GFP nonspecific signal (means +- SEM, n = 4 independ
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
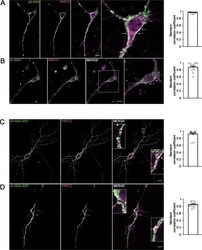
- Fig. 2 Co-localization of alpha3-NKA and alpha1-NKA with PRRT2 in primary hippocampal neurons. A , B Hippocampal neurons (DIV 14) were stained for endogenous PRRT2 and either alpha3-NKA ( A ) or alpha1-NKA ( B ). Left: Representative confocal images show the overlapping staining of NKA isoforms and PRRT2 (magnified in the insets). Scale bar, 10 mum. Right: Quantitative evaluation of the extent of the codistribution between PRRT2 and NKA isoforms using the Manders' correlation coefficient expressing the PRRT2/NAK overlap area in percent of the NAK immunoreactive area (0.96 +- 0.06 and 0.87 +- 0.04 for alpha3-NKA/PRRT2 and alpha1-NKA/PRRT2, respectively; n = 15 images from three independent experiments). C , D Hippocampal neurons transfected with PRRT2-HA and either alpha3-NKA-SEP ( C ) or alpha1-NKA-SEP ( D ) were live-labeled (DIV 14) with anti-HA and anti-GFP antibodies to stain the surface-exposed domains of the proteins. Left : The images show the largely overlapping staining of PRRT2 with both alpha3-NKA-SEP and alpha1-NKA-SEP (magnified in the insets). Scale bar, 10 mum. Right: Quantitative evaluation of the extent of the codistribution between PRRT2-HA and the NKA-SEP isoforms using the Manders' correlation coefficient expressing the PRRT2/NAK overlap area in percent of the area of NAK fluorescence (0.91 +- 0.04 and 0.84 +- 0.03 for alpha3-NKA/PRRT2 and alpha1-NKA/PRRT2, respectively; n = 15 images from three independent experiments).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig. 3 PRRT2 does not affect NKA expression level at the membrane surface. A , B Left: Representative immunoblots of cell surface biotinylation performed in WT and PRRT2 KO hippocampal neurons. Total lysates (TOTAL), biotinylated (cell surface, EXTRA), and non-biotinylated (intracellular, INTRA) fractions were analyzed by western blotting with antibodies to PRRT2 and either alpha3-NKA ( A ) or alpha1-NKA ( B ). Antibodies to transferrin receptor (Transf Rec) and actin were used as markers of cell surface and intracellular fractions, respectively. Vertical lines in the blot indicate that the lanes were on the same gel but have been repositioned in the figure. Right: Total and cell surface alpha3-NKA ( A ) and alpha1-NKA ( B ) immunoreactivities are expressed in percent of the respective WT value after normalization to Transferrin receptor (for the EXTRA fraction) or actin (for the INTRA fraction). Means +- SEM of n = 3 independent experiments; unpaired Mann-Whitney's U -test. C , D Left : Representative images of a WT neuron transfected with alpha3-NKA-SEP ( C ) or alpha1-NKA-SEP ( D ) perfused with Tyrode followed by MES buffers (scale bar, 20 mum). Insets show higher magnification of the fluorescence at the plasma membrane where ROIs were measured. The cartoons shown on top depict the SEP state under Tyrode and MES buffers, respectively. The green stars represent fluorescent emission of the chimeric protein, which is quenched at acidic pH, i.e., in acidic intracellular compa
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
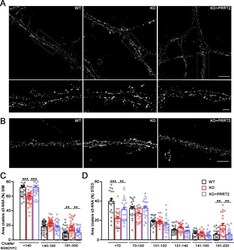
- Fig. 4 PRRT2 affects alpha3-NKA clustering at the membrane surface. A , B Representative 3D-SIM ( A ) and STED ( B ) images of hippocampal neurons (DIV 14-17) stained for alpha3-NKA. The images highlight an altered clustering of alpha3-NKA along the plasma membrane in PRRT2 KO neurons as compared to WT neurons that is rescued by expression of PRRT2 in PRRT2 KO neurons (KO + PRRT2). Scale bar, 5 mum; insets: 1 mum. C , D Size distribution of alpha3-NKA membrane clusters. Clusters were subdivided into bins on the basis of their size as indicated. The histograms (means +- SEM) show the area occupied by each bin of alpha3-NKA clusters expressed in percent of the total immunoreactive area. Both 3D-SIM ( C ) and STED ( D ) microscopy showed a significant increase in the area of largest clusters and a reciprocal decrease in the area of smallest clusters along the soma and the dendritic membrane in PRRT2 KO neurons (red bars) with respect to WT neurons (black bars). The change was fully rescued by re-expression of PRRT2 in PRRT2 KO neurons (KO + PRRT2, blue bars). Data refer to n = 35 (3D-SIM) and 25 (STED) neurons per genotype, from n = 3 independent preparations (3 coverslips/preparation/genotype). ** p < 0.01, *** p < 0.001; one-way ANOVA/Bonferroni tests.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
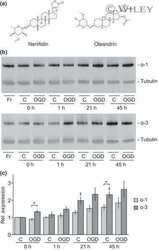
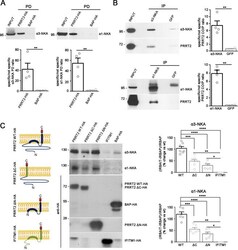
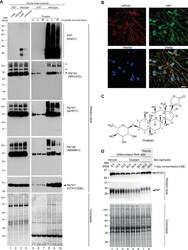
- 10.1371/journal.pone.0258682.g004 Fig 4 Validation of NKA binding to PrP. (A) Western blot-based validation of co-immunoprecipitation of NKA subunits with PrP. (B) Evidence of partial cellular co-localization of PrP C and ATP1A1 by co-immunocytochemical analysis of ReN VM cells, with Hoechst stain serving as the nuclear counter stain. Scale bar: 10 nm. (C) Chemical structure of the NKA inhibitor ouabain. (D) Parallel ouabain concentration-dependent effects on NKA and PrP C levels. Exposure of differentiated ReN VM cells to ouabain at concentrations up to 50 nM causes a biphasic effect on PrP C and ATP1A1 levels, with concentrations up to 30 nM leading to a ouabain concentration-dependent reduction in steady-state levels of both proteins, and exposure to 50 nM provoking a reproducible slowing of PrP C during SDS-PAGE separation that parallels an increase in steady-state ATP1A1 levels.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
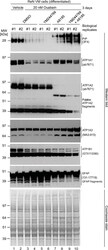
- 10.1371/journal.pone.0258682.g008 Fig 8 The steady-state levels of isoforms of PrP C and NKA alpha-subunits are individually controlled by extracellular CGs and intracellular Ca 2+ ions. Exposure to 20 nM ouabain caused a reduction in the steady-state levels of PrP C , ATP1A1 and ATP1A2--but not ATP1A3--in a manner that cannot be reversed by concomitant incubation with YM24476. In contrast, co-incubation of cells with ouabain and A6185 rescued the reduction in ATP1A1 levels and caused the appearance of signals detected with the PrP C - and ATP1A2-directed antibodies that migrated with different apparent molecular weights than the dominant signals for these proteins observed in untreated cells. Finally, the intensity of relatively fast migrating signals detected with a GFAP-directed antibody increase under conditions that favor intracellular calcium-dependent calpain activity.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
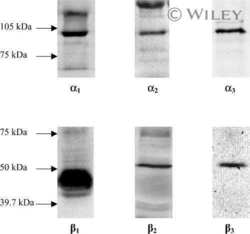
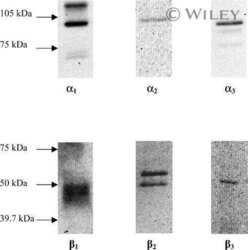
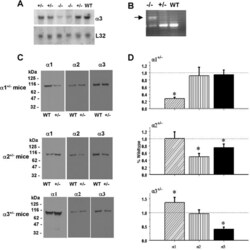
- Figure 1. Na,K-ATPase isoforms are reduced in gene-targeted mice. A , Reduced Na,K-ATPase alpha3 isoform mRNA expression in embryonic E18.5 d brains from WT, heterozygous (+/-), and homozygous (-/-) alpha3 knock-out mice by Northern blot. L32 was used as a loading control. Twenty micrograms of total RNA were loaded per lane. B , RT-PCR analysis of RNA from WT, heterozygous (+/-), and homozygous (-/-) alpha3 knock-out mice shows larger RNA transcript in -/- (arrow) but not +/- or WT mice. C , Western blot analysis of Na,K-ATPase isoforms in whole tissue extracts from hippocampus of adult male alpha1 +/- , alpha2 +/- , and alpha3 +/- mice. Total protein loaded per lane was as follows: 10, 0.5, and 1 mug for alpha1, alpha2, and alpha3 isoform expression, respectively. D , Semiquantitation by densitometry on whole tissue extracts from adult hippocampus of alpha1 +/- , alpha2 +/- , and alpha3 +/- mice shows reduction in alpha1, alpha2, and alpha3 isoforms, respectively. * p < 0.05 versus WT.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
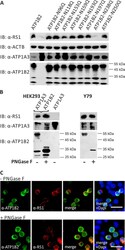
- Fig 2 Retinoschisin binding in dependence of ATP1B2 glycosylation. (A) HEK293 cells were co-transfected with expression constructs for ATP1A3 and expression constructs for ATP1B2 mutants deficient in each one glycosylation site (ATP1B2-N96Q, -N118Q, -N153Q, -N159Q, -N193Q, -N197Q, -N238Q, and -N250Q). 48 h after transfection, cells were incubated with recombinant retinoschisin for 1 h, followed by intensive washing. Cells transfected with only ATP1A3 expression constructs served as a negative control, cells co-transfected with expression constructs for ATP1A3 and for normal ATP1B2 served as a positive control in the retinoschisin binding assay [ 13 ]. Heterologous protein expression as well as retinoschisin binding was investigated by Western blot analyses with antibodies against retinoschisin, ATP1A3, and ATP1B2. The ACTB staining served as loading control. (B) Enriched membrane fractions from HEK293 cells transfected with ATP1A3 and ATP1B2 expression constructs and Y79 cells were subjected to enzymatic deglycosylation using PNGase F. A control sample was subjected to the same treatment, but without PNGase F. Subsequently, membrane fractions were incubated with recombinant retinoschisin for 1 h, followed by intensive washing. Retinoschisin binding, (heterologous) Na/K-ATPase expression, and ATP1B2 deglycosylation were investigated by Western blot analyses with antibodies against retinoschisin, ATP1A3, and ATP1B2. Membranes from HEK293 cells transfected with only ATP1A3 e
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
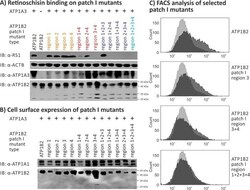
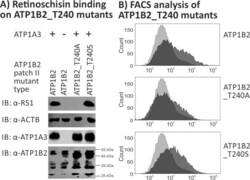
- Fig 4 Retinoschisin binding to ATP1B2 patch I mutants. HEK293 cells were transfected with expression constructs for ATP1A3 in combination with expression constructs for different ATP1B2 variants mutated in patch I regions depicted within the figure. (A) 48 h after transfection, cells were subjected to recombinant retinoschisin for 1 h, followed by intensive washing. Cells transfected with expression constructs for only ATP1B2 served as a negative control, cells transfected with expression constructs for ATP1A3 and normal ATP1B2 served as a positive control in the retinoschisin binding assay [ 13 ]. Heterologous protein expression as well as retinoschisin binding was investigated by Western blot analyses with antibodies against retinoschisin, ATP1A3, and ATP1B2. The ACTB staining served as loading control. (B) 48 h after transfection, cell surface proteins were biotinylated, purified by streptavidin affinity chromatography and analysed by Western blotting using antibodies against ATP1B2. The ATP1A1 immunoblot was performed as loading control. (C) 48 h after transfection, cells were subjected to FACS analyses applying an anti-ATP1B2 antibody. Representative histograms of cells transfected with selected ATP1B2 patch I mutants are given in this figure. Light grey: histogram of untransfected HEK293 cells depicting unspecific background signals. Dark grey: histogram of transfected HEK293 cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Fig 6 Retinoschisin binding to ATP1B2_T240A and ATP1B2_T240S. HEK293 cells were transfected with expression constructs for ATP1A3 in combination with expression constructs for ATP1B2_T240A and ATP1B2_T240S. (A) 48 h after transfection, cells were subjected to recombinant retinoschisin for 1 h, followed by intensive washing. Cells transfected with expression constructs for only ATP1B2 served as a negative control, cells transfected with expression constructs for ATP1A3 and normal ATP1B2 served as a positive control in the retinoschisin binding assay [ 13 ]. Heterologous protein expression as well as retinoschisin binding was investigated by Western blot analyses with antibodies against retinoschisin, ATP1A3, and ATP1B2. The ACTB staining served as loading control. (B) 48 h after transfection, cells were subjected to FACS analyses applying an anti-ATP1B2 antibody, representative histograms are given in this figure. Light grey: histogram of untransfected HEK293 cells depicting unspecific background signals. Dark grey: histogram of transfected HEK293 cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
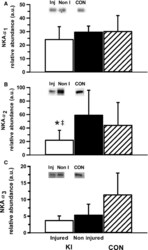
- Experimental details
- Figure 2 Muscle NKA alpha 1 (A), alpha 2 (B), and alpha 3 (C) isoform relative abundance from the vastus lateralis of the injured and noninjured legs of participants with knee injury (KI) and from a single leg of age- and BMI-matched controls (CON). Representative western blots are included; ""Inj"" represents the knee-injured leg, ""Non-I"" represents the noninjured leg, and ""CON"" represents the control group. Unfilled bars represent the injured leg from KI, filled bars represent the noninjured leg from KI and the hatched bars represent CON. *Less than noninjured leg ( P < 0.05), ‡ Moderate effect size compared to noninjured leg. Values are Mean +- SD, n = 6 for each leg KI, n = 7 CON.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
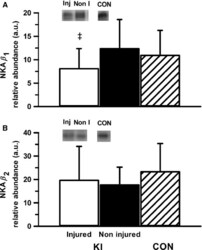
- Experimental details
- Figure 3 Muscle NKA beta 1 (A) and beta 2 (B) isoform relative abundance from the vastus lateralis of the injured and noninjured legs of participants with knee injury (KI) and from a single leg of age- and BMI-matched controls (CON). Representative western blots are included; Inj represents the knee-injured leg, Non-I represents the noninjured leg, and CON represents the control group. Hollow bars represent the injured leg from KI, filled bars represent the noninjured leg from KI and the hatched bars represent the CON. ‡ Moderate effect size compared to noninjured leg in KI. Values are Mean +- SD, n = 6 for each leg KI, n = 7 CON.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- FIGURE 4 Validation of the identified proteins in dendritic filopodia. (A,B) Localization of the identified proteins in dendritic filopodia (A1-A21) and phagocytic cups (B1-B21) at 14 DIV hippocampal neurons. The cultured neurons were immunostained with anti-TLCN antibody and specific antibodies against Galphao (A1,B1) , Na + /K + ATPase alpha3 (A2,B2) , EFA6D (A3,B3 ), spectrin (A4,B4) , septin7 (A5,B5) , myosin VA (A6,B6) , Galphaq (A7,B7) , Gbeta2 (A8,B8) , eEF1gamma (A9,B9) , ribosomal protein S16 (A10,B10) , CaMKIIalpha (A11,B11) , CD98hc (A12,B12) , EFA6C (A13,B13) , BAIAP2L1 (A14,B14) , PLCbeta3 (A15,B15) , MRCKalpha (A16,B16) , NR3A/B (GluN3A/B) (A17,B17) , SAP97 (SLC3A2) (A18,B18) , MAP1S (A19,B19) , EPS8L1 (A20,B20) , and JIP-4 (SPAG9) (A21,B21) . In merged images, the identified proteins and TLCN are shown in magenta and green, respectively. Single plane of images focused on dendritic filopodia (A1-A21) and center of microbeads (B1-B21) were acquired using a confocal microscopy. Scale bars, 5 mum in (A14,A21,B14,B21) .
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunocytochemistry
Immunocytochemistry Flow cytometry
Flow cytometry