44-750G
antibody from Invitrogen Antibodies
Targeting: MAPT
DDPAC, FLJ31424, FTDP-17, MAPTL, MGC138549, MSTD, MTBT1, MTBT2, PPND, PPP1R103, tau
Antibody data
- Antibody Data
- Antigen structure
- References [51]
- Comments [0]
- Validations
- Immunohistochemistry [2]
- Flow cytometry [1]
- Other assay [33]
Submit
Validation data
Reference
Comment
Report error
- Product number
- 44-750G - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- Phospho-Tau (Ser262) Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- The antibody has been negatively preadsorbed using a non-phosphopeptide corresponding to the site of phosphorylation to remove antibody that is reactive with non-phosphorylated Tau. The final product is generated by affinity chromatography using a Tau-derived peptide that is phosphorylated at serine 262. Positive control used: Recombinant human Tau +/- PKA, or African green monkey kidney (CV-1) cells, stably expressing human four repeat Tau and SV40 small T antigen to induce specific inhibition of PP2A.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Storage
- -20°C
Submitted references Toxicities of amyloid-beta and tau protein are reciprocally enhanced in the Drosophila model.
Identification of a reciprocal negative feedback loop between tau-modifying proteins MARK2 kinase and CBP acetyltransferase.
Sustained Hippocampal Synaptic Pathophysiology Following Single and Repeated Closed-Head Concussive Impacts.
Loganin substantially ameliorates molecular deficits, pathologies and cognitive impairment in a mouse model of Alzheimer's disease.
PKR kinase directly regulates tau expression and Alzheimer's disease-related tau phosphorylation.
Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis.
IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer's disease.
Mixed pathologies in pancreatic β cells from subjects with neurodegenerative diseases and their interaction with prion protein.
Melatonin directly binds and inhibits death-associated protein kinase 1 function in Alzheimer's disease.
Truncation of Tau selectively facilitates its pathological activities.
Effects of pharmacological modulators of α-synuclein and tau aggregation and internalization.
Brain-specific repression of AMPKα1 alleviates pathophysiology in Alzheimer's model mice.
Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion.
The KLDpT activation loop motif is critical for MARK kinase activity.
Caffeine Consumption During Pregnancy Accelerates the Development of Cognitive Deficits in Offspring in a Model of Tauopathy.
Pathological Tau From Alzheimer's Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in vivo.
Amylin as a potential link between type 2 diabetes and alzheimer disease.
GSKIP-Mediated Anchoring Increases Phosphorylation of Tau by PKA but Not by GSK3beta via cAMP/PKA/GSKIP/GSK3/Tau Axis Signaling in Cerebrospinal Fluid and iPS Cells in Alzheimer Disease.
α-lipoic acid attenuates spatial learning and memory impairment induced by hepatectomy.
Subacute to chronic Alzheimer-like alterations after controlled cortical impact in human tau transgenic mice.
Isozyme-Specific Role of SAD-A in Neuronal Migration During Development of Cerebral Cortex.
Involvement of Activation of Asparaginyl Endopeptidase in Tau Hyperphosphorylation in Repetitive Mild Traumatic Brain Injury.
The Isoquinoline Alkaloid Dauricine Targets Multiple Molecular Pathways to Ameliorate Alzheimer-Like Pathological Changes In Vitro.
Isoflurane anesthesia in aged mice and effects of A1 adenosine receptors on cognitive impairment.
Rac1 activation links tau hyperphosphorylation and Aβ dysmetabolism in Alzheimer's disease.
Stabilization of dynamic microtubules by mDia1 drives Tau-dependent Aβ(1-42) synaptotoxicity.
A Dual Pathogenic Mechanism Links Tau Acetylation to Sporadic Tauopathy.
Tau deletion promotes brain insulin resistance.
The Deacetylase HDAC6 Mediates Endogenous Neuritic Tau Pathology.
Elucidation of Dietary Polyphenolics as Potential Inhibitor of Microtubule Affinity Regulating Kinase 4: In silico and In vitro Studies.
Specific ion channels contribute to key elements of pathology during secondary degeneration following neurotrauma.
Beta-amyloid 1-42 monomers, but not oligomers, produce PHF-like conformation of Tau protein.
Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer's disease treatment.
Tau hyperphosphorylation in synaptosomes and neuroinflammation are associated with canine cognitive impairment.
Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer's Disease Pathology and Improves Cognition in the 5XFAD Mouse.
Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer's disease brain.
Specificity of anti-tau antibodies when analyzing mice models of Alzheimer's disease: problems and solutions.
Tau pathogenesis is promoted by Aβ1-42 but not Aβ1-40.
Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function.
Microtubule-associated protein tau in bovine retinal photoreceptor rod outer segments: comparison with brain tau.
An experimental rat model of sporadic Alzheimer's disease and rescue of cognitive impairment with a neurotrophic peptide.
Asp664 cleavage of amyloid precursor protein induces tau phosphorylation by decreasing protein phosphatase 2A activity.
Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo.
Transgenic mouse and cell culture models demonstrate a lack of mechanistic connection between endoplasmic reticulum stress and tau dysfunction.
Developmental regulation of tau phosphorylation, tau kinases, and tau phosphatases.
Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells.
Kinase activities increase during the development of tauopathy in htau mice.
Suppression of tubulin polymerization by the LKB1-microtubule-associated protein/microtubule affinity-regulating kinase signaling.
Increased tau phosphorylation on mitogen-activated protein kinase consensus sites and cognitive decline in transgenic models for Alzheimer's disease and FTDP-17: evidence for distinct molecular processes underlying tau abnormalities.
Pseudophosphorylation of tau protein alters its ability for self-aggregation.
Pseudophosphorylation of tau protein alters its ability for self-aggregation.
Sun ZD, Hu JX, Wu JR, Zhou B, Huang YP
Neural regeneration research 2022 Oct;17(10):2286-2292
Neural regeneration research 2022 Oct;17(10):2286-2292
Identification of a reciprocal negative feedback loop between tau-modifying proteins MARK2 kinase and CBP acetyltransferase.
Tabassum Z, Tseng JH, Isemann C, Tian X, Chen Y, Herring LE, Cohen TJ
The Journal of biological chemistry 2022 Jun;298(6):101977
The Journal of biological chemistry 2022 Jun;298(6):101977
Sustained Hippocampal Synaptic Pathophysiology Following Single and Repeated Closed-Head Concussive Impacts.
McDaid J, Briggs CA, Barrington NM, Peterson DA, Kozlowski DA, Stutzmann GE
Frontiers in cellular neuroscience 2021;15:652721
Frontiers in cellular neuroscience 2021;15:652721
Loganin substantially ameliorates molecular deficits, pathologies and cognitive impairment in a mouse model of Alzheimer's disease.
Nie L, He K, Xie F, Xiao S, Li S, Xu J, Zhang K, Yang C, Zhou L, Liu J, Zou L, Yang X
Aging 2021 Oct 23;13(20):23739-23756
Aging 2021 Oct 23;13(20):23739-23756
PKR kinase directly regulates tau expression and Alzheimer's disease-related tau phosphorylation.
Reimer L, Betzer C, Kofoed RH, Volbracht C, Fog K, Kurhade C, Nilsson E, Överby AK, Jensen PH
Brain pathology (Zurich, Switzerland) 2021 Jan;31(1):103-119
Brain pathology (Zurich, Switzerland) 2021 Jan;31(1):103-119
Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis.
Lee HJ, Jung YH, Choi GE, Kim JS, Chae CW, Lim JR, Kim SY, Yoon JH, Cho JH, Lee SJ, Han HJ
Cell death and differentiation 2021 Jan;28(1):184-202
Cell death and differentiation 2021 Jan;28(1):184-202
IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer's disease.
Brigas HC, Ribeiro M, Coelho JE, Gomes R, Gomez-Murcia V, Carvalho K, Faivre E, Costa-Pereira S, Darrigues J, de Almeida AA, Buée L, Dunot J, Marie H, Pousinha PA, Blum D, Silva-Santos B, Lopes LV, Ribot JC
Cell reports 2021 Aug 31;36(9):109574
Cell reports 2021 Aug 31;36(9):109574
Mixed pathologies in pancreatic β cells from subjects with neurodegenerative diseases and their interaction with prion protein.
Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, Marcilla I, Marti-Andres G, Caballero MC, Riverol M, Tuñon MT, Fraser PE, Luquin MR
Acta neuropathologica communications 2021 Apr 8;9(1):64
Acta neuropathologica communications 2021 Apr 8;9(1):64
Melatonin directly binds and inhibits death-associated protein kinase 1 function in Alzheimer's disease.
Chen D, Mei Y, Kim N, Lan G, Gan CL, Fan F, Zhang T, Xia Y, Wang L, Lin C, Ke F, Zhou XZ, Lu KP, Lee TH
Journal of pineal research 2020 Sep;69(2):e12665
Journal of pineal research 2020 Sep;69(2):e12665
Truncation of Tau selectively facilitates its pathological activities.
Gu J, Xu W, Jin N, Li L, Zhou Y, Chu D, Gong CX, Iqbal K, Liu F
The Journal of biological chemistry 2020 Oct 2;295(40):13812-13828
The Journal of biological chemistry 2020 Oct 2;295(40):13812-13828
Effects of pharmacological modulators of α-synuclein and tau aggregation and internalization.
Dominguez-Meijide A, Vasili E, König A, Cima-Omori MS, Ibáñez de Opakua A, Leonov A, Ryazanov S, Zweckstetter M, Griesinger C, Outeiro TF
Scientific reports 2020 Jul 30;10(1):12827
Scientific reports 2020 Jul 30;10(1):12827
Brain-specific repression of AMPKα1 alleviates pathophysiology in Alzheimer's model mice.
Zimmermann HR, Yang W, Kasica NP, Zhou X, Wang X, Beckelman BC, Lee J, Furdui CM, Keene CD, Ma T
The Journal of clinical investigation 2020 Jul 1;130(7):3511-3527
The Journal of clinical investigation 2020 Jul 1;130(7):3511-3527
Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion.
Mankhong S, Kim S, Moon S, Lee KH, Jeon HE, Hwang BH, Beak JW, Joa KL, Kang JH
International journal of molecular sciences 2020 Aug 14;21(16)
International journal of molecular sciences 2020 Aug 14;21(16)
The KLDpT activation loop motif is critical for MARK kinase activity.
Sonntag T, Moresco JJ, Yates JR 3rd, Montminy M
PloS one 2019;14(12):e0225727
PloS one 2019;14(12):e0225727
Caffeine Consumption During Pregnancy Accelerates the Development of Cognitive Deficits in Offspring in a Model of Tauopathy.
Zappettini S, Faivre E, Ghestem A, Carrier S, Buée L, Blum D, Esclapez M, Bernard C
Frontiers in cellular neuroscience 2019;13:438
Frontiers in cellular neuroscience 2019;13:438
Pathological Tau From Alzheimer's Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in vivo.
Miao J, Shi R, Li L, Chen F, Zhou Y, Tung YC, Hu W, Gong CX, Iqbal K, Liu F
Frontiers in aging neuroscience 2019;11:34
Frontiers in aging neuroscience 2019;11:34
Amylin as a potential link between type 2 diabetes and alzheimer disease.
Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, Riverol M, Marcilla I, de Andrea CE, Sánchez-Arias JA, Del Mar Carmona-Abellan M, Marti G, Erro ME, Martínez-Vila E, Tuñon MT, Luquin MR
Annals of neurology 2019 Oct;86(4):539-551
Annals of neurology 2019 Oct;86(4):539-551
GSKIP-Mediated Anchoring Increases Phosphorylation of Tau by PKA but Not by GSK3beta via cAMP/PKA/GSKIP/GSK3/Tau Axis Signaling in Cerebrospinal Fluid and iPS Cells in Alzheimer Disease.
Ko HJ, Chiou SJ, Wong YH, Wang YH, Lai Y, Chou CH, Wang C, Loh JK, Lieu AS, Cheng JT, Lin YT, Lu PJ, Fann MJ, Huang CF, Hong YR
Journal of clinical medicine 2019 Oct 21;8(10)
Journal of clinical medicine 2019 Oct 21;8(10)
α-lipoic acid attenuates spatial learning and memory impairment induced by hepatectomy.
Zhang Y, Lv YL, Si YN, Zhou J, Qian Y, Bao HG
Experimental and therapeutic medicine 2019 Mar;17(3):2329-2333
Experimental and therapeutic medicine 2019 Mar;17(3):2329-2333
Subacute to chronic Alzheimer-like alterations after controlled cortical impact in human tau transgenic mice.
Zhang Y, Wu F, Iqbal K, Gong CX, Hu W, Liu F
Scientific reports 2019 Mar 7;9(1):3789
Scientific reports 2019 Mar 7;9(1):3789
Isozyme-Specific Role of SAD-A in Neuronal Migration During Development of Cerebral Cortex.
Nakanishi K, Niida H, Tabata H, Ito T, Hori Y, Hattori M, Johmura Y, Yamada C, Ueda T, Takeuchi K, Yamada K, Nagata KI, Wakamatsu N, Kishi M, Pan YA, Ugawa S, Shimada S, Sanes JR, Higashi Y, Nakanishi M
Cerebral cortex (New York, N.Y. : 1991) 2019 Aug 14;29(9):3738-3751
Cerebral cortex (New York, N.Y. : 1991) 2019 Aug 14;29(9):3738-3751
Involvement of Activation of Asparaginyl Endopeptidase in Tau Hyperphosphorylation in Repetitive Mild Traumatic Brain Injury.
Hu W, Tung YC, Zhang Y, Liu F, Iqbal K
Journal of Alzheimer's disease : JAD 2018;64(3):709-722
Journal of Alzheimer's disease : JAD 2018;64(3):709-722
The Isoquinoline Alkaloid Dauricine Targets Multiple Molecular Pathways to Ameliorate Alzheimer-Like Pathological Changes In Vitro.
Liu P, Chen X, Zhou H, Wang L, Zhang Z, Ren X, Zhu F, Guo Y, Huang X, Liu J, Spencer PS, Yang X
Oxidative medicine and cellular longevity 2018;2018:2025914
Oxidative medicine and cellular longevity 2018;2018:2025914
Isoflurane anesthesia in aged mice and effects of A1 adenosine receptors on cognitive impairment.
Zuo CL, Wang CM, Liu J, Shen T, Zhou JP, Hao XR, Pan YZ, Liu HC, Lian QQ, Lin H
CNS neuroscience & therapeutics 2018 Mar;24(3):212-221
CNS neuroscience & therapeutics 2018 Mar;24(3):212-221
Rac1 activation links tau hyperphosphorylation and Aβ dysmetabolism in Alzheimer's disease.
Borin M, Saraceno C, Catania M, Lorenzetto E, Pontelli V, Paterlini A, Fostinelli S, Avesani A, Di Fede G, Zanusso G, Benussi L, Binetti G, Zorzan S, Ghidoni R, Buffelli M, Bolognin S
Acta neuropathologica communications 2018 Jul 13;6(1):61
Acta neuropathologica communications 2018 Jul 13;6(1):61
Stabilization of dynamic microtubules by mDia1 drives Tau-dependent Aβ(1-42) synaptotoxicity.
Qu X, Yuan FN, Corona C, Pasini S, Pero ME, Gundersen GG, Shelanski ML, Bartolini F
The Journal of cell biology 2017 Oct 2;216(10):3161-3178
The Journal of cell biology 2017 Oct 2;216(10):3161-3178
A Dual Pathogenic Mechanism Links Tau Acetylation to Sporadic Tauopathy.
Trzeciakiewicz H, Tseng JH, Wander CM, Madden V, Tripathy A, Yuan CX, Cohen TJ
Scientific reports 2017 Mar 13;7:44102
Scientific reports 2017 Mar 13;7:44102
Tau deletion promotes brain insulin resistance.
Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, Issad T, Gerhardt E, Pagesy P, Vileno M, Bournonville C, Hamdane M, Bantubungi K, Lancel S, Demeyer D, Eddarkaoui S, Vallez E, Vieau D, Humez S, Faivre E, Grenier-Boley B, Outeiro TF, Staels B, Amouyel P, Balschun D, Buee L, Blum D
The Journal of experimental medicine 2017 Aug 7;214(8):2257-2269
The Journal of experimental medicine 2017 Aug 7;214(8):2257-2269
The Deacetylase HDAC6 Mediates Endogenous Neuritic Tau Pathology.
Tseng JH, Xie L, Song S, Xie Y, Allen L, Ajit D, Hong JS, Chen X, Meeker RB, Cohen TJ
Cell reports 2017 Aug 29;20(9):2169-2183
Cell reports 2017 Aug 29;20(9):2169-2183
Elucidation of Dietary Polyphenolics as Potential Inhibitor of Microtubule Affinity Regulating Kinase 4: In silico and In vitro Studies.
Khan P, Rahman S, Queen A, Manzoor S, Naz F, Hasan GM, Luqman S, Kim J, Islam A, Ahmad F, Hassan MI
Scientific reports 2017 Aug 25;7(1):9470
Scientific reports 2017 Aug 25;7(1):9470
Specific ion channels contribute to key elements of pathology during secondary degeneration following neurotrauma.
O'Hare Doig RL, Chiha W, Giacci MK, Yates NJ, Bartlett CA, Smith NM, Hodgetts SI, Harvey AR, Fitzgerald M
BMC neuroscience 2017 Aug 14;18(1):62
BMC neuroscience 2017 Aug 14;18(1):62
Beta-amyloid 1-42 monomers, but not oligomers, produce PHF-like conformation of Tau protein.
Manassero G, Guglielmotto M, Zamfir R, Borghi R, Colombo L, Salmona M, Perry G, Odetti P, Arancio O, Tamagno E, Tabaton M
Aging cell 2016 Oct;15(5):914-23
Aging cell 2016 Oct;15(5):914-23
Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer's disease treatment.
Sun W, Lee S, Huang X, Liu S, Inayathullah M, Kim KM, Tang H, Ashford JW, Rajadas J
Scientific reports 2016 Oct 6;6:34784
Scientific reports 2016 Oct 6;6:34784
Tau hyperphosphorylation in synaptosomes and neuroinflammation are associated with canine cognitive impairment.
Smolek T, Madari A, Farbakova J, Kandrac O, Jadhav S, Cente M, Brezovakova V, Novak M, Zilka N
The Journal of comparative neurology 2016 Mar 1;524(4):874-95
The Journal of comparative neurology 2016 Mar 1;524(4):874-95
Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer's Disease Pathology and Improves Cognition in the 5XFAD Mouse.
Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, Liu Y, Terry AV Jr, Bieberich E
The Journal of neuroscience : the official journal of the Society for Neuroscience 2016 Aug 17;36(33):8653-67
The Journal of neuroscience : the official journal of the Society for Neuroscience 2016 Aug 17;36(33):8653-67
Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer's disease brain.
Takeda S, Wegmann S, Cho H, DeVos SL, Commins C, Roe AD, Nicholls SB, Carlson GA, Pitstick R, Nobuhara CK, Costantino I, Frosch MP, Müller DJ, Irimia D, Hyman BT
Nature communications 2015 Oct 13;6:8490
Nature communications 2015 Oct 13;6:8490
Specificity of anti-tau antibodies when analyzing mice models of Alzheimer's disease: problems and solutions.
Petry FR, Pelletier J, Bretteville A, Morin F, Calon F, Hébert SS, Whittington RA, Planel E
PloS one 2014;9(5):e94251
PloS one 2014;9(5):e94251
Tau pathogenesis is promoted by Aβ1-42 but not Aβ1-40.
Hu X, Li X, Zhao M, Gottesdiener A, Luo W, Paul S
Molecular neurodegeneration 2014 Nov 23;9:52
Molecular neurodegeneration 2014 Nov 23;9:52
Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function.
Oaks AW, Frankfurt M, Finkelstein DI, Sidhu A
PloS one 2013;8(4):e60378
PloS one 2013;8(4):e60378
Microtubule-associated protein tau in bovine retinal photoreceptor rod outer segments: comparison with brain tau.
Yamazaki A, Nishizawa Y, Matsuura I, Hayashi F, Usukura J, Bondarenko VA
Biochimica et biophysica acta 2013 Oct;1832(10):1549-59
Biochimica et biophysica acta 2013 Oct;1832(10):1549-59
An experimental rat model of sporadic Alzheimer's disease and rescue of cognitive impairment with a neurotrophic peptide.
Bolognin S, Blanchard J, Wang X, Basurto-Islas G, Tung YC, Kohlbrenner E, Grundke-Iqbal I, Iqbal K
Acta neuropathologica 2012 Jan;123(1):133-51
Acta neuropathologica 2012 Jan;123(1):133-51
Asp664 cleavage of amyloid precursor protein induces tau phosphorylation by decreasing protein phosphatase 2A activity.
Park SS, Jung HJ, Kim YJ, Park TK, Kim C, Choi H, Mook-Jung IH, Koo EH, Park SA
Journal of neurochemistry 2012 Dec;123(5):856-65
Journal of neurochemistry 2012 Dec;123(5):856-65
Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo.
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA, Yu WH, Duff KE
Autophagy 2012 Apr;8(4):609-22
Autophagy 2012 Apr;8(4):609-22
Transgenic mouse and cell culture models demonstrate a lack of mechanistic connection between endoplasmic reticulum stress and tau dysfunction.
Spatara ML, Robinson AS
Journal of neuroscience research 2010 Jul;88(9):1951-61
Journal of neuroscience research 2010 Jul;88(9):1951-61
Developmental regulation of tau phosphorylation, tau kinases, and tau phosphatases.
Yu Y, Run X, Liang Z, Li Y, Liu F, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX
Journal of neurochemistry 2009 Mar;108(6):1480-94
Journal of neurochemistry 2009 Mar;108(6):1480-94
Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells.
Ojala JO, Sutinen EM, Salminen A, Pirttilä T
Journal of neuroimmunology 2008 Dec 15;205(1-2):86-93
Journal of neuroimmunology 2008 Dec 15;205(1-2):86-93
Kinase activities increase during the development of tauopathy in htau mice.
Kelleher I, Garwood C, Hanger DP, Anderton BH, Noble W
Journal of neurochemistry 2007 Dec;103(6):2256-67
Journal of neurochemistry 2007 Dec;103(6):2256-67
Suppression of tubulin polymerization by the LKB1-microtubule-associated protein/microtubule affinity-regulating kinase signaling.
Kojima Y, Miyoshi H, Clevers HC, Oshima M, Aoki M, Taketo MM
The Journal of biological chemistry 2007 Aug 10;282(32):23532-40
The Journal of biological chemistry 2007 Aug 10;282(32):23532-40
Increased tau phosphorylation on mitogen-activated protein kinase consensus sites and cognitive decline in transgenic models for Alzheimer's disease and FTDP-17: evidence for distinct molecular processes underlying tau abnormalities.
Lambourne SL, Sellers LA, Bush TG, Choudhury SK, Emson PC, Suh YH, Wilkinson LS
Molecular and cellular biology 2005 Jan;25(1):278-93
Molecular and cellular biology 2005 Jan;25(1):278-93
Pseudophosphorylation of tau protein alters its ability for self-aggregation.
Haase C, Stieler JT, Arendt T, Holzer M
Journal of neurochemistry 2004 Mar;88(6):1509-20
Journal of neurochemistry 2004 Mar;88(6):1509-20
Pseudophosphorylation of tau protein alters its ability for self-aggregation.
Haase C, Stieler JT, Arendt T, Holzer M
Journal of neurochemistry 2004 Mar;88(6):1509-20
Journal of neurochemistry 2004 Mar;88(6):1509-20
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
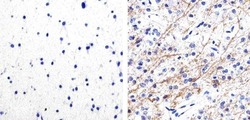
- Experimental details
- Immunohistochemistry analysis of Phospho-Tau (pS262) showing staining in the cytoplasm of paraffin-embedded human astroglioma tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Phospho-Tau (pS262) polyclonal antibody (Product # 44-750G) diluted in 3% BSA-PBS at a dilution of 1:100 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
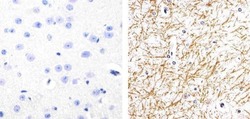
- Experimental details
- Immunohistochemistry analysis of Phospho-Tau (pS262) showing staining in the cytoplasm of paraffin-embedded mouse brain tissue (right) compared to a negative control without primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 min. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 min at room temperature, washed with ddH2O and PBS, and then probed with a Phospho-Tau (pS262) polyclonal antibody (Product # 44-750G) diluted in 3% BSA-PBS at a dilution of 1:20 overnight at 4ºC in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
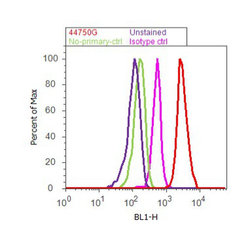
- Experimental details
- Flow cytometry analysis of Tau [pS262] was done on SH-SY5Y cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with Tau [pS262] Rabbit Polyclonal Antibody (44750G, red histogram) or with rabbit isotype control (pink histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
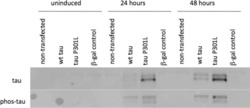
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
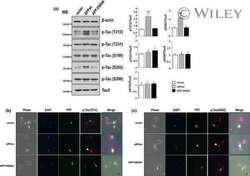
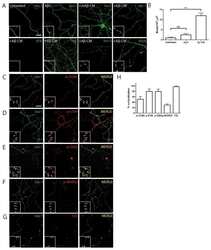
- Figure 6 Screen for additional pJNK-Tau sites in striatum. (A) Phosphorylation of Tau protein at epitopes subject to phosphorylation by JNK was analyzed by immunoblot. (B) Band optical density (OD) from phosphorylation-specific probes relative to total Tau expression is presented as percent of 2 month-old WT (mean +- SEM) and was analyzed by two-way ANOVA with Bonferroni post-hoc tests comparing each A53T group to age-matched controls (*p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
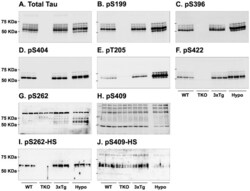
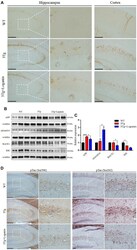
- Figure 7 Analysis of tau signal with polyclonal antibodies by Western blotting. Proteins were extracted from the cortex of 3 mouse lines: control mice (WT and Hypothermic), Tau KO mice and 3xTg-AD mice. Proteins were separated by SDS-PAGE and then identified with the following polyclonal antibodies: A: Total Tau, B: pS199, C: pS396, D: pS404, E: pT205, F: pS422, G: pS262 and H: pS409. Normal anti-rabbit secondary antibodies were used to detect primary antibodies. The heat stable fraction was used to remove non-specificity: I: pS262 and J: pS409. Quantifications of the blots are available in Figure S5 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
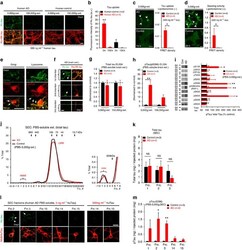
- Figure 6 Neuronal uptake of PBS-soluble HMW tau derived from human AD brain. ( a , b ) Primary neurons were incubated with AD or control brain extracts (cases were matched for age and postmortem interval ( Supplementary Table S1 )) and immunostained at day 2 ( a ). ( b ) Quantification of fluorescence intensity of human tau staining. One-way ANOVA and a subsequent Tukey-Kramer test. ( c , d ) Tau uptake ( c ) and seeding activity ( d ) assay in HEK-tau-biosensor cells. (Mann-Whitney U -test) ( e ) Subcellular localization of human tau taken up by neurons (PBS-3,000 g , 500 ng ml -1 human tau). ( f ) Neuron-to-neuron transfer of tau in a 3-chamber microfluidic device. AD brain extract (PBS-3,000 g , 500 ng ml -1 human tau) was added to the 1st chamber. Human tau positive neurons were detected in both the 1st and 2nd chamber at day 7 (arrow). ( g , h ) Quantification of total-tau ( g ) and phospho-tau ( h ) levels in AD and control brain extract (ELISA). Unpaired t -test. ( i ) Brain extracts were immunoblotted with phospho-tau specific antibodies recognizing different epitopes. Representative immunoblot and quantification of phospho-tau levels at each epitope. Unpaired t -test. ( j , k ) SEC analysis of PBS-soluble tau from AD and control brain. ( j ) Representative graph of total tau levels (ELISA) in SEC-separated samples. Small peaks for HMW fractions were detected in both groups (right panel). ( k ) Mean total tau levels of HMW SEC fractions. ( l ) Tau uptake from each SEC
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
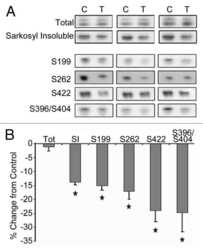
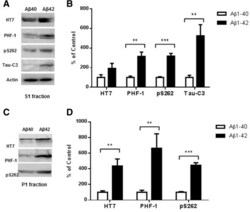
- Figure 5 Higher level of total tau, phospho-tau and cleaved tau in P301S mice receiving an Abeta 1-42 injection into the entorhinal cortex as compared with Abeta 1-40 injection. 8 month old P301S mice were injected with Abeta1-40 into the left entorhinal cortex and Abeta1-42 into the right entorhinal cortex. 18 days after injection, the brain extracts were analyzed by western blot. A) Representative images of western blots of soluble tau (S1 fraction) detected with anti-tau antibodies and normalized to actin. B) Quantification of western blots in (A) . C) Representative images of western blots of insoluble tau (P1 fraction) detected with anti-tau antibodies. Both S1 brain fraction and P1 brain fraction show a significant increase in HT7, PHF-1 and pS262 signal in entorhinal cortex injected with Abeta1-42 compared with Abeta1-40 injected entorhinal cortex. Also more cleaved tau (Tau421) was detected in the S1 fraction from the Abeta1-42 injected brain. D) Quantification of western blots in (C) . Histographs show mean +/- standard error, n =3. *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
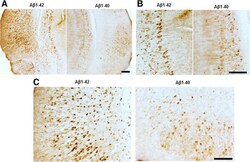
- Figure 6 Abeta 1-42 treatment increases pathological tau recognized by AT180 when compared with Abeta 1-40 treatment. Brain sections were immunostained with AT180, which recognizes p-tau at Thr 231 . (A) Representative images for pathological tau detected with AT180. (B) Higher magnification showed significantly increased tau tangles in Abeta1-42 injected entorhinal cortex (C) and hippocampus (B) . Magnification: 4x for A; 10x for B and C. Scale bar: 100um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
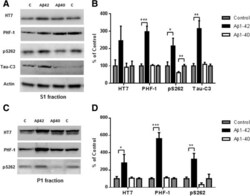
- Figure 7 Abeta 1-42 promotes tau phosphorylation, cleavage and aggregation while Abeta 1-40 does not. 8 month old P301S mice (1N4R form with Prion promoter) were injected with saline into the left entorhinal cortex and Abeta1-42 (2ug) into the right entorhinal cortex or saline into the left entorhinal cortex and Abeta1-40 (2ug) into the right entorhinal cortex. 18 days after injection, the brain extracts were analyzed by western blot. A) Representative images of western blots of soluble tau (S1 fraction) detected with anti-tau antibodies and normalized to actin. B) Quantification of western blots in (A) . C) Representative images of western blots of insoluble tau (P1 fraction), detected with anti-tau antibodies. D) Quantification of western blots in (C) Abeta1-42 injection significantly induced tau phosphorylation (PHF-1 and pS262 epitope) in the S1 and P1 brain fractions. 3-fold increase in Tau421 was found in the soluble fraction (A and B) . Significant decrease in pS262 immunoreactivity was observed in S1 fraction from Abeta1-40 injected brain areas compared to control side. Histographs show mean +/- standard error, n =3. *p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
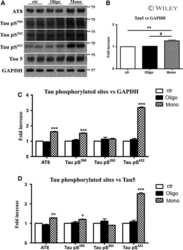
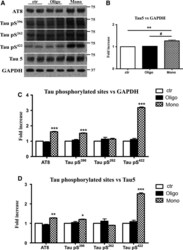
- Figure 3 Abeta1-42 monomers promote phosphorylation at particular sites that have been related to Alzheimer's disease (AD) progression. Representative Western blot of brain extracts (20 mug protein) from control (saline) and treated (Abeta1-42 peptides by ICV for 3 h) mice using antibodies specific for the detection four pathological Tau phosphorylation sites: AT8, pS396, pS262, and pS422. An antibody raised against GAPDH or Tau 5 served as loading control. Densitometric quantification shows an increase of the total protein level of AT8, pS396, and pS422 induced by monomers while monomeric and oligomeric preparations did not change pS262 expression. The data are mean +- standard error of the mean (SEM), * P < 0.05; *** P < 0.001 vs. control by one-way ANOVA followed by Bonferroni post hoc test, n = 6.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
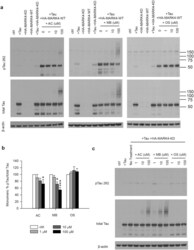
- Figure 3 MB reduced Tau phosphorylation by overexpressed MARK4 in 293T cells. ( a ) Representative immunoblot of lysates from 293T cells transfected with 4R2N Tau alone, HA-MARK4 (KD and WT) alone or Tau + HA-MARK4. Cells transfected with Tau + HA-MARK4-WT were treated with AC, MB and OS for 3 h at concentrations indicated in the panel. The levels of pTau S262 and total Tau were reported with beta-actin as the loading control. ( b ) Quantification of the ratio of monomeric pTau S262 to monomeric total Tau presented as the percent of the level found in cells transfected with Tau and MARK4-WT but without drug treatment. Results are mean +- SD (n = 3). *0.01 < P < 0.05; ***P < 0.001. Student's t test is conducted between corresponding values in AC and OS or MB and OS group. ( c ) Representative immunoblot of lysates from 293T cells transfected with Tau + HA-MARK4-KD followed by treatment with AC, MB and OS as described in A). beta-actin blots were cropped. Full blots are shown in Supplementary Fig. S4 .
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
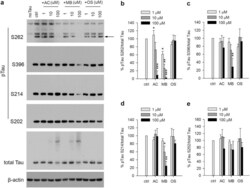
- Figure 4 MB reduced Tau phosphorylation by endogenous kinases in 293T cells. ( a ) Representative immunoblot of lysates from 293T cells transfected with 4R2N Tau. Transfected cells were treated with AC, MB and OS for 3 h at concentrations indicated in the panel. The levels of pTau S262, S396, S214, S202 and total Tau were reported with beta-actin as the loading control. ( b-e ) Quantification of the ratio of pTau to total Tau presented as the percent of the level found in cells transfected with Tau but without drug treatment. Results are mean +- SD (n = 3). *0.01 < P < 0.05; **0.001 < P < 0.01 ***P < 0.001. Student's t test is conducted between corresponding values in AC and OS or MB and OS group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image

- Experimental details
- Figure 6 MB directly inhibits the ability of MARK4 to phosphorylate Tau in an in vitro kinase assay. GST-MARK4 was incubated with purified His-Tau and ATP in the presence of increasing concentration of AC, MB or OS for 30 min at 30 degC. ( a ) Representative immunoblot of the kinase reaction probed by antibodies against GST, pTau S262 and total Tau. ( b ) Quantification of the ratio of monomeric pTau S262 to monomeric total Tau presented as the percent of the level without drug treatment. Results are mean +- SD (n = 4). *0.01 < P < 0.05; **0.001 < P < 0.01. Student's t test is conducted between corresponding values in AC and OS or MB and OS group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
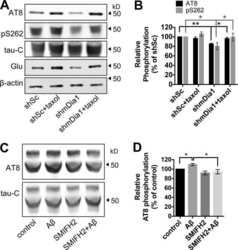
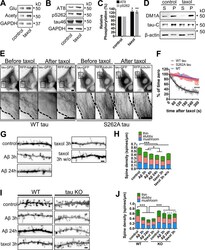
- Figure 8. Inhibition of MT dynamics causes tau hyperphosphorylation and spine loss though tau. (A and B) WB analysis of Glu and acetylated (Acety) tubulin (A) as well as phosphorylated (AT8 and pS262) and total (tau-46) tau levels (B) in hippocampal neurons (7 DIV) incubated with 10 nM taxol for 3 h. (C) Quantification of normalized AT8 and pS262 against control levels in taxol-treated neurons. (D) WB analysis of total tau (tau-C) and tubulin (DM1A) levels in cortical neurons (7 DIV) treated with 10 nM taxol for 3 h. Aliquots of soluble (S) and MT pellet (P) fractions are shown. (E) Representative images from time-lapse recordings of NIH3T3 cells cotransfected with human 2N4R WT or S262A tau-GFP and RFP-tubulin before and 10 min after 10 nM taxol. Dashed lines indicate cell peripheries. Bar, 20 um. (F) Quantification of background-subtracted MT-bound WT tau, S262A tau, and MT intensity after taxol treatment. (G and H) Representative images (G) and quantification (H) of spine density and morphology of hippocampal neurons (21 DIV) treated with vehicle control, Abeta, or taxol for the indicated times +- washout (w/o). (I and J) Representative images (I) and quantification (J) of spine density and morphology of hippocampal neurons (21 DIV) from WT and tau-KO mice treated with vehicle control, Abeta, or taxol for the indicated times. Bars, 2 um. Gray asterisks in J compare Abeta- or taxol-treated KO neurons to KO control neurons. Data are shown as means +- SEM. *, P < 0.5; **,
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
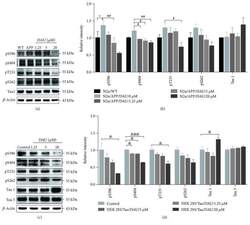
- Figure 3 DAU attenuated tau phosphorylation in both N2a/APP cells and HEK293/Tau cells. Levels of phosphorylated tau and total tau in N2a/WT and N2a/APP cells (a, b) and in HEK293/Tau cells (c, d) as determined by Western blot analysis. beta -Actin was used as a loading control. N = 3. Data show the mean +- SEM. * P < 0.05 compared to N2a/WT cells, # P < 0.05 and ## P < 0.01 compared to untreated N2a/APP cells, and & P < 0.05 and &&& P < 0.001 compared to untreated HEK293/Tau cells.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
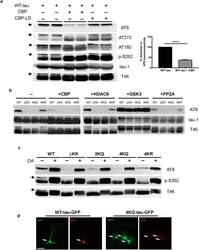
- Experimental details
- Figure 1 Tau acetylation modulates tau phosphorylation at specific epitopes. (a-c ) Immunoblot analysis and quantification using the indicated tau antibodies for (a ) cells expressing WT tau-T40 and either an active form of CBP or a catalytically inactive form of CBP (CBP-LD), ( b ) cells co- expressing tau-T40 (WT, DeltaKK, 4KQ, or 4KR) and tau modifying enzymes, and ( c ) cells expressing tau-T40 (WT, DeltaKK, 2KQ, 4KQ, or 4KR) and treated with control (DMSO) or okadaic acid (OA), where indicated. Solid black arrows highlight the ~75 kDa phospho-tau species that is reduced upon acetylation. Statistical significance was assessed using a student t-test (****p < 0.0001). Cropped images from full size immunoblots are provided in panels a-c. Full-length immunoblots are presented in Supplementary Fig. S1 . ( d ) Immunofluorescence microscopy of primary cortical neurons expressing WT-tau-GFP (left) or 4KQ-tau-GFP (right) detecting phosphorylated tau (AT8). White arrows identify transfected neurons. Scale bar, 50 um.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
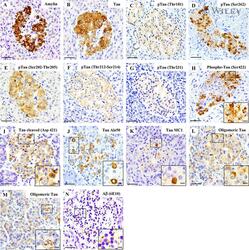
- Experimental details
- Amylin, tau, and Abeta inclusions in pancreatic beta cells. Immunohistochemistry is shown for amylin (A), total tau (B), Thr181 phosphorylated tau (AT270; C), Ser262 phosphorylated tau (D), Ser202-Thr205 phosphorylated tau (AT8; E), Thr212-Ser214 phosphorylated tau (AT100; F), Thr231 phosphorylated tau (AT180; G), Ser422 phosphorylated tau (H), Asp421 cleaved tau (I), tau Alz50 (J), tau MC-1 (K), endocrine oligomeric tau (L), exocrine oligomeric tau (M), and Abeta inclusions (N) in pancreatic cells from a 75-year-old male with Alzheimer disease and no history of type 2 diabetes mellitus. Magnification = x40, scale bars = 50mum. The boxed areas (H-N) were examined at a higher magnification: x63 magnification, scale bars = 20mum.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
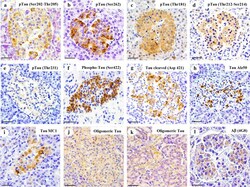
- Experimental details
- Fig. 3 Tau and Abeta inclusions in pancreatic beta cells of subjects with Parkinson''s disease, Lewy body dementia and incidental Lewy body disease. Immunohistochemistry for Ser202-Thr205-phosphorylated tau (AT8) ( a ), Ser262-phosphorylated tau ( b ), Thr181-phosphorylated tau (AT270) ( c ), Thr212-Ser214-phosphorylated tau (AT100) ( d ), Thr231-phosphorylated tau (AT180) ( e ), Ser422-phosphorylated tau ( f ), Asp421-cleaved tau ( g ), tau Alz50 ( h ), tau MC-1 ( i ), endocrine oligomeric tau ( j ), exocrine oligomeric tau ( k ) and Abeta ( l ) inclusions in pancreatic cells from subjects with incidental Lewy body disease ( a , b , c , f ), Parkinson''s disease ( d , e , g , h , i , l ) and dementia with Lewy body ( j , k ): a 40 x magnification; scale bar = 50 um
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
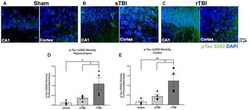
- Experimental details
- Figure 6 Hippocampal and cortical phosphorylated tau is elevated after single (sTBI) or repeated TBI (rTBI). (A-C) Representative confocal images (63x, multi-plane) show immunolabeling of phosphorylated tau (pTau S262, green) within the CA1 region of the hippocampus (left) and Layer V of the cortex (right) ipsilateral to injury in sham (A) , sTBI (B) , and rTBI (C) animals. (D) Bar graphs show a significant increase in hippocampal phosphorylated tau burden in the rTBI group (black) relative to the sTBI (gray) and sham groups (white; n = 16 slices/4 rats for each treatment condition). (E) Bar graphs show a significant increase in cortical phosphorylated tau burden in the rTBI group (black) relative to the sTBI (gray) and sham groups (white; n = 16 slices/4 rats for each treatment condition). Representative images were obtained on a Leica Microsystems SP8 confocal microscope. The injury was over the right sensorimotor cortex. * p
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
- Figure 2 Effects of loganin on AD pathology and the expression of Abeta-related key proteins. ( A ) The relative expression of Abeta deposition. ( B , C ) The relative expression of APP expression and processing related proteins. ( D ) The relative expression of Tau phosphorylation. Scale bar = 100 mum. Data were expressed as mean +- SEM, * p < 0.05, *** p < 0.001, n = 3 for each group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
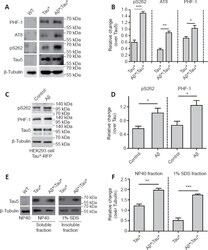
- Amyloid-beta (Abeta) aggravates tau protein hyperphosphorylation and accumulation. (A) Western blot analysis of tau phosphorylation using phosphorylation site-specific antibodies. Protein extracts were prepared from 25 adult fly heads. ELAV-Gal4 was used to drive Tau* and Abeta* expression in the fly central nervous system (CNS). Tubulin was used as a loading control. (B) The quantitative results for panel A. The pS262, PHF-1, and AT8 levels were normalized to the Tau-5 antibody signal. (C) Western blot analysis of tau hyperphosphorylation in HEK293 cells with Tau*-RFP expression. Tau*-RFP cells were treated with 10 muM Abeta 40 peptide for 24 hours. Tubulin was used as the loading control. (D) The quantitative results for panel C. Western blot results were calculated using ImageJ software. Levels of pS262 and PHF-1 were normalized to tau5. (E) Western blot analysis of the levels of soluble and insoluble tau protein. Tubulin was used as the loading control. Protein extracts were prepared from 50 adult fly heads. ELAV-Gal4 was used to drive Tau* and Abeta* expression in the fly CNS. (F) The quantitative results for panel E. The tau protein level was normalized to tubulin. Data are presented as the mean +- SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 (unpaired t -test). All western blot analyses were performed three times, and representative blots are shown.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
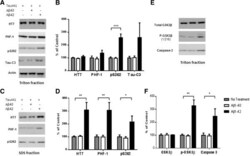
- Figure 3 A beta1-42 promotes phosphorylation, cleavage and aggregation of tau by increasing GSK3beta activity and by activating pro-caspase3. Sequential extraction was performed on Tau441-YFP-transfected cells treated with transfection reagent alone (control), 200 nM Abeta1-40 or Abeta1-42 for 24 h. Triton fraction was obtained by extracting intracellular tau with 1% Triton. Triton-insoluble fraction was then solubilized with 1% SDS to get SDS fraction. A) Triton fraction was electrophoresed and immunoblotted for total tau (HT7), p-tau(Ser 396/404 ) (PHF-1), p-tau(Thr 262 ) (pS262), Tau421(tau-C3) and beta-actin. beta-Actin was measured from the same blots to ensure that equal protein was present in every lane. Abeta1-42 significantly induced p-tau at Thr 231 and increased levels of Tau421 were observed in Abeta1-42 treated cells, while no change occurred in Abeta1-40 treated cells when compared to cells without treatment. B) Quantification of western blots in (A) . C) Representative images of western blots for SDS fraction with HT7, PHF-1 and pS262. All 3 forms of tau increased in Abeta1-42 treated cells. No change in tau level was found in Abeta1-40 treated cells. There was no detectable signal for tau-C3 in SDS fraction. D) Quantification of western blots in (C) . E) Triton fraction was also blotted with anti-GSK3beta antibody, anti-p-GSK3beta (Y216) and anti-caspase-3 antibody. Abeta1-42 treatment resulted in an increase in phosphorylation of GSK3beta, which represents hi
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
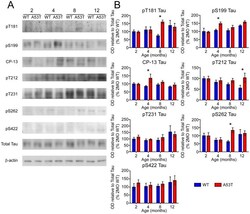
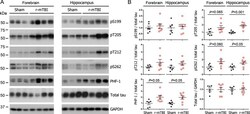
- Fig. 1 Tau phosphorylation increases at multiple sites in the brain following r-mTBI in 3xTg-AD mice. Adult female 3xTg-AD mice, 4-- months of age, were subjected to repetitive mild closed-head brain impacts, totaling five with 48-h inter-impact intervals, and sacrificed 24 h after the final impact. A) Representative western blots showing the level of indicated proteins/phosphorylation sites. B) Densitometric quantification of the blots. Among the phosphorylation sites examined, tau was hyperphosphorylated at pThr 205 , pSer 262 and PHF-1 (pSer 396/404 ) sites. Data are presented as scatter dot plots with mean+-SEM ( n = 6-7 mice each) and analyzed using unpaired t test, with Welch's correction in the case of unequal variance.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
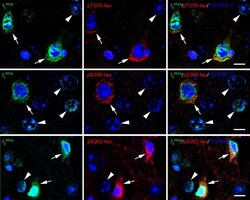
- Fig. 5 Translocated I 2 PP2A /SET is colocalized with hyperphosphorylated tau in the neuronal cytoplasm in cerebral cortex of 3xTg-AD mice with r-mTBI. Brain sections were fluorescently dual immuno-stained for I 2 PP2A and phospho-tau. Translocated I 2 PP2A colocalized with pThr 205 -tau, pSer 396 -tau and with pSer 262 -tau in the cytoplasm of neurons (arrows). However, there was no somatodendritic phospho-tau staining in neurons where cytoplasmic I 2 PP2A was absent (filled arrowheads). Scale bar = 10 mu m for all images.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
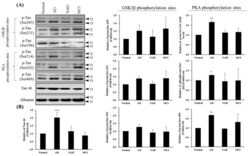
- Experimental details
- Figure 4 Western blot of Tau46 (total Tau) and phosphorylated Tau by GSK3beta and PKA kinases from cerebrospinal fluid (CSF) sampling of four groups: normal, Alzheimer's disease (AD), neurological disorders (NAD), and mild cognitive impairment (MCI). ( A ) Western blotting with Tau 46, Tau Ser205, Tau Ser231, and Tau Ser396 by GSK3beta or Tau Ser214, Tau Ser262, and Tau Ser409 by PKA antibodies. Albumin was used as an internal control. ( B ) Statistical analysis. Bar graphs represent the mean +- SD of triplicates. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group.
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
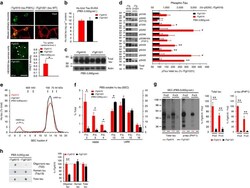
- Experimental details
- Figure 2 Lack of PBS-soluble phosphorylated HMW tau species is associated with low tau uptake in primary neurons. ( a , top) Uptake of human tau from brain extracts from rTg4510 and rTg21221 mice by primary neurons (PBS-3,000 g , 500 ng ml -1 human tau). Neurons were immunostained with human tau-specific antibody (green) and total (human and mouse) tau antibody (red). ( a , bottom) Tau uptake assay in HEK-tau-biosensor cells. Brain extracts (10 mug protein) were applied to the cells (lipofectamie (-)). ( n =4) Unpaired t -test. Scale bar, 50 mum. ( b ) Human tau levels in brain extracts (ELISA). ( c ) Immunoblot analysis of PBS-soluble extracts with total tau antibody (DA9). Up-shifted bands in rTg4510 brain suggest phosphorylation of tau (arrow). ( d ) Brain extracts were immunoblotted with phospho-tau specific antibodies recognizing different epitopes. Representative immunoblot and quantification of phospho-tau levels at each epitope. ( n =3-4) Unpaired t -test. ( e , f ) SEC analysis of PBS-soluble tau. ( e ) Representative graph of human tau levels (ELISA) in SEC-separated samples ( f ) Mean human tau levels of HMW (Frc. 2-4) and LMW (Frc. 13-16) SEC fractions. ( n =3-6) Unpaired t -test. ( g ) Immunoblot analysis (SDS-PAGE) of SEC-separated fractions from brain extracts (total tau, DAKO). Quantification of band density is also shown (right graphs) ( n =4). Unpaired t -test. ( h ) Dot blot analysis of PBS-soluble brain extracts with tau oligomer-specific antibody (T22), h
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
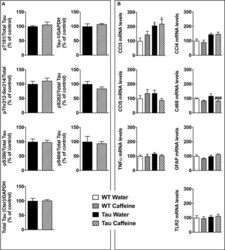
- Experimental details
- FIGURE 5 Investigation of early-life exposure to caffeine on hippocampal Tau phosphorylation and associated neuroinflammatory markers. (A) Western blot analysis of tau phosphorylation in water or caffeine exposed THY-Tau22 offsprings using antibodies targeting physiological (pThr181, Tau-1, pSer262, pSer396, pSer404) and pathologic (pThr212/Ser214) Tau epitopes. Quantifications were performed over total tau levels (Cter). Total tau levels were quantified versus GAPDH, used as loading control ( n = 7/group). (B) qPCR analysis of hippocampal neuroinflammatory markers associated with Tau pathology in the THY-Tau22 model ( n = 6-10 per group).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry