Antibody data
- Antibody Data
- Antigen structure
- References [13]
- Comments [0]
- Validations
- Flow cytometry [1]
- Other assay [7]
Submit
Validation data
Reference
Comment
Report error
- Product number
- PA1-048 - Provider product page

- Provider
- Invitrogen Antibodies
- Product name
- alpha-2a Adrenergic Receptor Polyclonal Antibody
- Antibody type
- Polyclonal
- Antigen
- Synthetic peptide
- Description
- PA1-048 detects alpha-2A adrenergic receptor (A2AAR) from human, rat and mouse tissues. PA1-048 has been successfully used in Western blot and immunohistochemistry procedures. By Western blot, this antibody detects an ~45 kDa protein representing A2AAR from mouse kidney membrane preparations. The PA1-048 immunogen is a synthetic peptide corressponding to residues R(218) I Y Q I A K R R T R V P P S R R G(235) of the 3rd intracellular loop of human A2AAR. This sequence is completely conserved between human, mouse, rat, and porcine A2AAR.
- Reactivity
- Human, Mouse, Rat
- Host
- Rabbit
- Isotype
- IgG
- Vial size
- 100 μL
- Concentration
- 0.6 mg/mL
- Storage
- -20°C, Avoid Freeze/Thaw Cycles
Submitted references Carotid smooth muscle contractility changes after severe burn.
Zerumbone Modulates α(2A)-Adrenergic, TRPV1, and NMDA NR2B Receptors Plasticity in CCI-Induced Neuropathic Pain In Vivo and LPS-Induced SH-SY5Y Neuroblastoma In Vitro Models.
Circuit-specific hippocampal ΔFosB underlies resilience to stress-induced social avoidance.
Norepinephrine Regulation of Adrenergic Receptor Expression, 5' AMP-Activated Protein Kinase Activity, and Glycogen Metabolism and Mass in Male Versus Female Hypothalamic Primary Astrocyte Cultures.
Brimonidine Protects Auditory Hair Cells from in vitro-Induced Toxicity of Gentamicin.
Single-cell analysis of long non-coding RNAs in the developing human neocortex.
Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex.
Immunohistochemical localization of α2-adrenergic receptors in the neonatal rat cochlea and the vestibular labyrinth.
Stimulation of α(2A)-adrenoceptors promotes the maturation of dendritic spines in cultured neurons of the medial prefrontal cortex.
Functional interaction between alpha2-adrenoceptors, mu- and kappa-opioid receptors in the guinea pig myenteric plexus: effect of chronic desipramine treatment.
Alpha2-adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents.
Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by alpha2-adrenoceptors in the presence of experimental colitis.
Leptin immunoreactivity in the central nervous system in normal and diabetic rats.
DeSpain K, Rosenfeld CR, Huebinger R, Wang X, Jay JW, Radhakrishnan RS, Wolf SE, Song J
Scientific reports 2021 Sep 10;11(1):18094
Scientific reports 2021 Sep 10;11(1):18094
Zerumbone Modulates α(2A)-Adrenergic, TRPV1, and NMDA NR2B Receptors Plasticity in CCI-Induced Neuropathic Pain In Vivo and LPS-Induced SH-SY5Y Neuroblastoma In Vitro Models.
Chia JSM, Izham NAM, Farouk AAO, Sulaiman MR, Mustafa S, Hutchinson MR, Perimal EK
Frontiers in pharmacology 2020;11:92
Frontiers in pharmacology 2020;11:92
Circuit-specific hippocampal ΔFosB underlies resilience to stress-induced social avoidance.
Eagle AL, Manning CE, Williams ES, Bastle RM, Gajewski PA, Garrison A, Wirtz AJ, Akguen S, Brandel-Ankrapp K, Endege W, Boyce FM, Ohnishi YN, Mazei-Robison M, Maze I, Neve RL, Robison AJ
Nature communications 2020 Sep 8;11(1):4484
Nature communications 2020 Sep 8;11(1):4484
Norepinephrine Regulation of Adrenergic Receptor Expression, 5' AMP-Activated Protein Kinase Activity, and Glycogen Metabolism and Mass in Male Versus Female Hypothalamic Primary Astrocyte Cultures.
Ibrahim MMH, Bheemanapally K, Sylvester PW, Briski KP
ASN neuro 2020 Jan-Dec;12:1759091420974134
ASN neuro 2020 Jan-Dec;12:1759091420974134
Brimonidine Protects Auditory Hair Cells from in vitro-Induced Toxicity of Gentamicin.
Cortada M, Levano S, Bodmer D
Audiology & neuro-otology 2017;22(3):125-134
Audiology & neuro-otology 2017;22(3):125-134
Single-cell analysis of long non-coding RNAs in the developing human neocortex.
Liu SJ, Nowakowski TJ, Pollen AA, Lui JH, Horlbeck MA, Attenello FJ, He D, Weissman JS, Kriegstein AR, Diaz AA, Lim DA
Genome biology 2016 Apr 14;17:67
Genome biology 2016 Apr 14;17:67
Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex.
Pollen AA, Nowakowski TJ, Shuga J, Wang X, Leyrat AA, Lui JH, Li N, Szpankowski L, Fowler B, Chen P, Ramalingam N, Sun G, Thu M, Norris M, Lebofsky R, Toppani D, Kemp DW 2nd, Wong M, Clerkson B, Jones BN, Wu S, Knutsson L, Alvarado B, Wang J, Weaver LS, May AP, Jones RC, Unger MA, Kriegstein AR, West JA
Nature biotechnology 2014 Oct;32(10):1053-8
Nature biotechnology 2014 Oct;32(10):1053-8
Immunohistochemical localization of α2-adrenergic receptors in the neonatal rat cochlea and the vestibular labyrinth.
Cai J, Li J, Mao Y, Bai X, Xu L, Wang H
Journal of molecular neuroscience : MN 2013 Nov;51(3):1010-20
Journal of molecular neuroscience : MN 2013 Nov;51(3):1010-20
Stimulation of α(2A)-adrenoceptors promotes the maturation of dendritic spines in cultured neurons of the medial prefrontal cortex.
Ren WW, Liu Y, Li BM
Molecular and cellular neurosciences 2012 Feb;49(2):205-16
Molecular and cellular neurosciences 2012 Feb;49(2):205-16
Functional interaction between alpha2-adrenoceptors, mu- and kappa-opioid receptors in the guinea pig myenteric plexus: effect of chronic desipramine treatment.
Canciani L, Giaroni C, Zanetti E, Giuliani D, Pisani R, Moro E, Trinchera M, Crema F, Lecchini S, Frigo G
European journal of pharmacology 2006 Dec 28;553(1-3):269-79
European journal of pharmacology 2006 Dec 28;553(1-3):269-79
Alpha2-adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents.
Kreiss C, Toegel S, Bauer AJ
American journal of physiology. Gastrointestinal and liver physiology 2004 Sep;287(3):G658-66
American journal of physiology. Gastrointestinal and liver physiology 2004 Sep;287(3):G658-66
Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by alpha2-adrenoceptors in the presence of experimental colitis.
Blandizzi C, Fornai M, Colucci R, Baschiera F, Barbara G, De Giorgio R, De Ponti F, Breschi MC, Del Tacca M
British journal of pharmacology 2003 May;139(2):309-20
British journal of pharmacology 2003 May;139(2):309-20
Leptin immunoreactivity in the central nervous system in normal and diabetic rats.
Li HY, Wang LL, Yeh RS
Neuroreport 1999 Feb 5;10(2):437-42
Neuroreport 1999 Feb 5;10(2):437-42
No comments: Submit comment
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
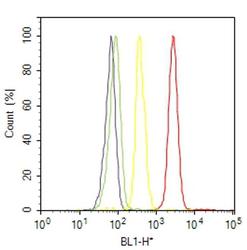
- Experimental details
- Flow cytometry analysis of alpha-2a Adrenergic Receptor was done on T-47D cells. Cells were fixed with 70% ethanol for 10 minutes, permeabilized with 0.25% Triton™ X-100 for 20 minutes, and blocked with 5% BSA for 30 minutes at room temperature. Cells were labeled with alpha-2a Adrenergic Receptor Rabbit Polyclonal Antibody (PA1048, red histogram) or with rabbit isotype control (yellow histogram) at 3-5 ug/million cells in 2.5% BSA. After incubation at room temperature for 2 hours, the cells were labeled with Alexa Fluor® 488 Goat Anti-Rabbit Secondary Antibody (A11008) at a dilution of 1:400 for 30 minutes at room temperature. The representative 10,000 cells were acquired and analyzed for each sample using an Attune® Acoustic Focusing Cytometer. The purple histogram represents unstained control cells and the green histogram represents no-primary-antibody control.
Supportive validation
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
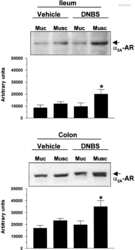
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
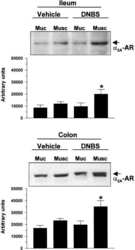
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
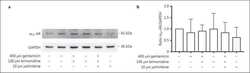
- Experimental details
- NULL
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
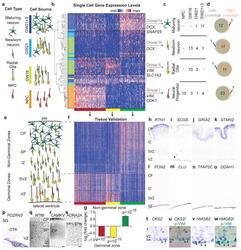
- Figure 3 Low-coverage single-cell mRNA sequencing distinguishes diverse neural cell types and identifies biomarkers in heterogeneous tissue. (a) Schematic of cell types and sources selected to represent stages of neuronal differentiation. Cultured neural progenitors represent early undifferentiated stages, while primary cortical samples are expected to contain radial glia, newborn, and maturing neurons. (b) Hierarchical clustering of 65 single cells across 500 genes with the strongest PC1-3 loading scores identifies four major groups of cells (I-IV) and k-means clustering identifies three clusters of genes (red, yellow, green). (c) Major groups can be interpreted based on the expression of known genes. Table shows the number of cells of specific types captured from each source. (d) Cell classification based on low-coverage data largely overlaps with classification based on high-coverage data. (e) Schematic of the distribution of cell types in developing cortex at mid-gestation. (f) Heatmap of gene expression values for PCA genes (columns) in 599 regions of the developing cortex 11 (rows). (g) Genes belonging to the red cluster (n = 218) and yellow cluster (n = 98) are enriched in the ventricular (VZ) and subventricular zones (SVZ), while genes belonging to the green cluster (n = 176) are enriched in the intermediate zone (IZ), subplate (SP), and cortical plate (CP); p values were calculated using Wilcoxon signed-rank test. (h-o) In situ hybridization for representative genes
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
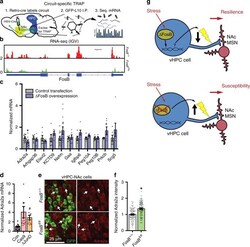
- Fig. 5 DeltaFosB orchestrates gene expression in NAc-projecting vHPC neurons. a Experimental design for circuit-specific TRAP. Retrograde Cre vector is injected into NAc and vHPC is harvested for immunoprecipitation of ribosomes from L10-GFP-expressing NAc-projecting cells and sequencing of actively translating mRNA. b Sequencing reads (red or green peaks) for FosB gene (exons indicated by thick blue bars beneath graph) from WT (FosB +/+ ) and FosB KO ( FosB fl/fl ) vHPC-NAc neuron mRNA. c DeltaFosB overexpression in Neuro2A cells regulates mRNA expression of some TRAP-identified target genes. * P = 0.0240 for Adra2a , # P = 0.0914 for Nefm , # P = 0.0815 for Prkcb (independent samples t -test compared to Control Transfection; Adra2a n = 12 plates/group; Arhgap36 n = 6; Elavl2 n = 6; Kctd9 n = 5-6; Nefm n = 12; Gaa n = 6; Igfbp6 n = 6; Peg10a n = 6; Peg10b n = 6; Prkcb n = 6; Scg5 n = 6). d Adra2a mRNA expression is increased in Neuro2A cells transfected with DeltaJunD or FosB -specific gRNA and Cas9 (Cas9). * P = 0.0133, # P = 0.0822 ( n = 12 wells/group; one-way ANOVA with Holm-Sidak post-tests versus control). e Representative coronal vHPC images (20X) showing NAc-projecting neurons expressing GFP (green) and alpha2AAR (red) in floxed FosB KO ( FosB fl/fl ; bottom panels) and WT ( FosB +/+ ; top panels) mice. f Intensity of alpha2AAR expression in NAc-projecting GFP-labeled is decreased by FosB KO ( FosB fl/fl ). * P < 0.0001 ( n = 100 FosB +/+ cells, n = 83 FosB fl/fl cel
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
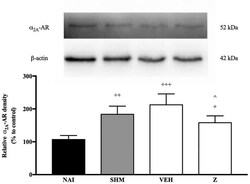
- Figure 7 Representative western blots of alpha 2A -adrenergic receptor from brain samples of naive, sham, vehicle and zerumbone-treated groups. Data presented as mean +- SEM (n = 4), which were normalized to beta-actin. + p < 0.05, ++ p < 0.01, +++ p < 0.001 as compared to naive and ^ p < 0.05 as compared to vehicle group. NAI (Naive); SHM (Sham); VEH (Vehicle, 10 mL/kg); Z (Zerumbone, 10 mg/kg).
- Submitted by
- Invitrogen Antibodies (provider)
- Main image
- Experimental details
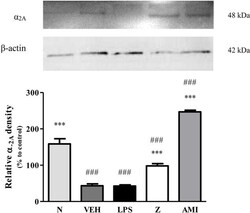
- Figure 12 Representative western blots of alpha 2A -adrenergic receptor from LPS-induced SH-SY5Y cells samples of normal, vehicle, LPS only, zerumbone, amitriptyline-treated groups. Data presented as mean +- SEM (n = 4), which were normalized to beta-actin. *** p < 0.001 as compared to LPS only group and ### p < 0.001 as compared to normal group. N (Normal); VEH (Vehicle, PBS); Z (Zerumbone, 8 mug/ml); AMI (Amitriptyline, 16 mug/ml).
 Explore
Explore Validate
Validate Learn
Learn Western blot
Western blot Immunohistochemistry
Immunohistochemistry Flow cytometry
Flow cytometry